Figure 1.
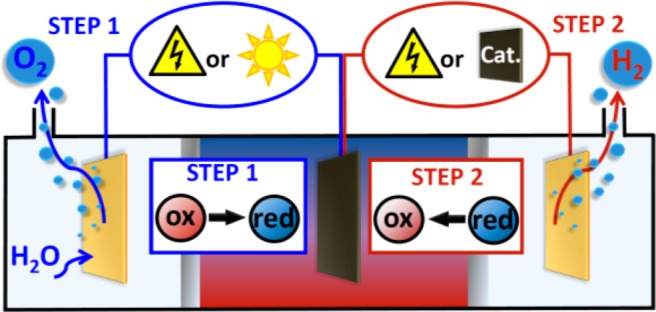
Use of ECPBs allows the electrolysis of water to be split into two steps both spatially and temporally. Step 1 (blue lines) is the oxidation of water concomitant with reduction of the ECPB, and step 2 (red lines) is reoxidation of the ECPB with concomitant hydrogen evolution. Previously, both steps have been driven electrochemically,8a,8b and an electrochemical step 1 followed by a catalytic release of H2 in step 2 has been demonstrated.8c Herein, we show that step 1 can be performed in a PEC, with an electrochemical step 2.
