Figure 2.
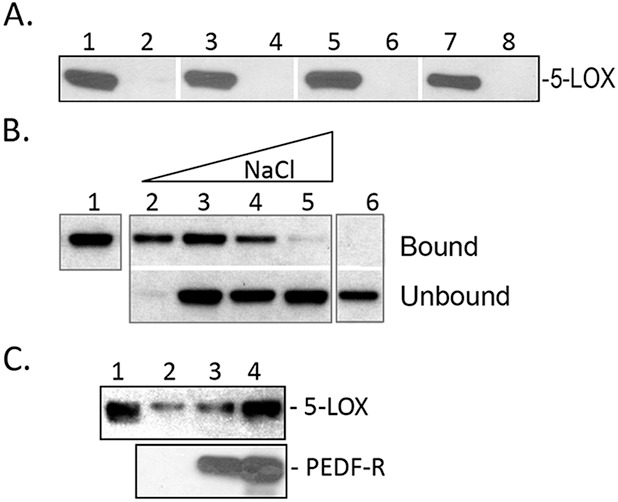
Binding of human recombinant 5-LOX to P1 peptide and PEDF-R. (A) Peptide-affinity chromatography of 5-LOX (300 ng) to P1 (0.4 nmol). Reactions were performed in 10 mM Tris-HCl, pH 7.5, under the following conditions: Lanes 1, 2, at 4°C, incubated for 60 minutes; lanes 3, 4, at 25°C, incubated for 15 minutes; lanes 5, 6, at 4°C, incubated for 60 minutes in buffer plus 3 mM DOC; lanes 7, 8, at 25°C, incubated for 15 minutes in buffer plus 3 mM DOC. After extensive washes with respective binding buffer, bound protein was eluted with SDS-PAGE sample buffer. Western blot versus anti-5-LOX is shown. Samples were loaded in gels as follows: Lanes 1, 3, 5, 7, bound to P1-agarose; lanes 2, 4, 6, 8, bound material to agarose without peptide. (B) Binding of 5-LOX (300 ng) to P1-affinity agarose column (0.4 nmol) in binding buffer (0.1 M sodium phosphate and 0.1% NP-40) with increasing NaCl concentrations. After extensive washes with binding buffer, bound protein was eluted with SDS-PAGE sample buffer. Lane 1, load; lane 2, 25 mM NaCl; lane 3, 50 mM NaCl; lane 4, 150 mM NaCl; lane 5, 250 mM NaCl; lane 6, 50 mM NaCl with control resin. Western blot versus anti-5-LOX is shown. (C) His-tag pull-down assays. Soluble fractions of cell-free expression reactions containing His6/Xpress-tagged PEDF-R at ∼350 and 700 ng (lanes 3, 4, respectively) were mixed with 5-LOX (250 ng) in binding buffer (0.1 M sodium phosphate, 50 mM NaCl, and 0.1% NP-40, 50 μL final volume) and incubated for 2 hours at 4°C with gentle rotation. Lane 2 was 5-LOX incubated with soluble fractions of cell-free expression reactions in the absence of PEDF-R. Then Ni-NTA resin beads (25 μL) were added, and the suspension was incubated for 1 hour at 4°C with gentle rotation. Bound 5-LOX (pull-down) was extracted with SDS sample buffer and analyzed by Western blot versus anti-5-LOX (top) and anti-PEDF-R (lower). Lane 1 was 5-LOX (250 ng) input.
