Abstract
Currently, chronic kidney disease (CKD) is a global health problem. Considering the impaired immunity of CKD patients, the relevance of infection in peritoneal dialysis (PD), and the increased prevalence of parasites in CKD patients, protozoa colonization was evaluated in PD effluent from CKD patients undergoing PD. Overnight PD effluent was obtained from 49 asymptomatic stable PD patients. Protozoa analysis was performed microscopically by searching cysts and trophozoites in direct wet mount of PD effluent and after staining smears. Protozoa were found in PD effluent of 10.2% of evaluated PD patients, namely Blastocystis hominis, in 2 patients, and Entamoeba sp., Giardia sp., and Endolimax nana in the other 3 patients, respectively. None of these patients presented clinical signs or symptoms of peritonitis at the time of protozoa screening. Our results demonstrate that PD effluent may be susceptible to asymptomatic protozoa colonization. The clinical impact of this finding should be further investigated.
Keywords: Peritoneal dialysis effluent, protozoa colonization, Blastocystis hominis, Entamoeba sp, Giardia sp, Endolimax nana
Alterations in immune response induced by the uremic state of chronic kidney disease (CKD) patients increase the susceptibility to infection and appear to play an important role in determining the type, incidence, and outcome of infectious complications (1,2). In this setting, opportunistic infections depend upon the state of immunosuppression and the epidemiological exposure of the patients. Despite prevention strategies, peritonitis remains a leading complication of peritoneal dialysis (PD). Therefore, prevention of infection is crucial for the success of PD therapy. The factors that influence the PD-related infections occurrence are still not entirely understood. Peritoneal dialysis-related peritonitis occur more frequently due to bacterial infection, but culture-negative peritoneal infection in some PD units still accounts for 20% of peritonitis episodes (3,4). In these culture-negative cases, consideration should be given to unusual microorganisms.
More than 4,000 million people worldwide are infected with parasitic infections (5). Nowadays, with the increase in number of patients with immune deficiency, increased organ transplantation, immune-suppressing drugs, and radiation therapy, higher statistics related to parasitic infection are common in developed countries (6). In accordance with the fact that parasitic infections are more prevalent in immunocompromised patients, several reports found higher prevalence of intestinal parasites in CKD patients than in healthy individuals (2,6). Although very scarce, some reports describe parasite infections in dialysis fluid in end-stage renal disease (ESRD) patients undergoing PD (7–9). Considering the low immunity of CKD patients, the high relevance of infection in PD, and the increased prevalence of parasites in CKD patients, the aim of the present study was to screen protozoa colonization in PD effluent from ESRD patients undergoing PD.
Materials and Methods
A group of 49 CKD patients undergoing PD in the outpatient clinic of the nephrology department of Centro Hospitalar S. João (Porto, Portugal) participated in the present study and their written informed consent was obtained (approved by the Ethics Committee of Centro Hospitalar de S. João). Peritoneal dialysis effluent (50 mL) was collected aseptically and centrifuged at 1,500 rpm, 10 minutes, 4°C. Six smears per patient were analyzed corresponding to 50 μL of pellet. For protozoa identification by microscopic evaluation, both latency and vegetative cellular forms were searched on direct wet mount smears with Lugols' iodine solution and stained with Giemsa, Trichrome, and Modified Acid-Fast Kinyoun (10). Protozoa evaluation in saliva and feces was performed as described for PD effluent. Feces were also analyzed by the local microbiology department. Feces were collected only from patients with PD effluent protozoa colonization.
Results and Discussion
The mean age of the 49 asymptomatic stable PD patients was 47.7 ± 12.9 years with a balanced proportion of each genus (55.1% male [M], 44.9% female [F]). In general, the evaluated population presented a low educational level (illiterate 4.1%, elementary school 53.0%, high school 6.1%, university 8.6%, unknown 22.4%). The most prevalent CKD etiologies were chronic glomerulonephritis (22.4%, including high prevalence of IgA nephropathy) and diabetic nephropathy (16.3%), followed by polycystic kidney disease (10.2%), others (26.5%), and undetermined (24.5%). These patients presented a mean time in a PD program of 10.8 ± 13.7 months, ranging from 1 to 72.3 months, with 89.8% on continuous ambulatory PD (CAPD) and 10.2% on automated PD (APD). From the 49 PD patients, 26.5% had 1 or more previous peritonitis episodes, bacteria and fungi being responsible for 70.6% and 5.9%, respectively, whereas culture-negative accounted for 23.5% of the total peritonitis episodes. Among 49 CKD patients undergoing PD, 5 presented PD effluent protozoa colonization without any clinical signs or symptoms of peritonitis. These 5 PD patients did not present leukocytosis or eosinophilia. In the PD effluent of 3 PD patients, cysts were observed, namely, 1 patient with cysts of Endolimax nana (Figure 1A) and 2 patients with cysts of Blastocystis hominis (Figure 1D). Also, we found throphozoites of Entamoeba sp. (Figure 1C) in 1 PD patient and throphozoites of Giardia sp. (Figure 1B) in another patient. In each positive PD patient sample, we observed less than 5 cellular forms (cysts or trophozoite) per smear. This may be due to the low number of protozoa found in each patient that can represent a non-infectious dose. However, even if these protozoa are considered symbionts, it should be noted that this population is immune-impaired, and thus commensals may represent relevant opportunistic microorganisms. In the few clinical reports describing the presence of parasites in dialysis effluent, a Pseudomonas sp. peritonitis with concomitant presence of a protozoon, Balantidium hominis, was described (9). The patient was treated efficiently with specific antibiotic therapy for Pseudomonas sp. infection, raising the question of the role of this specific protozoan: symbiont or pathogen.
Figure 1 —
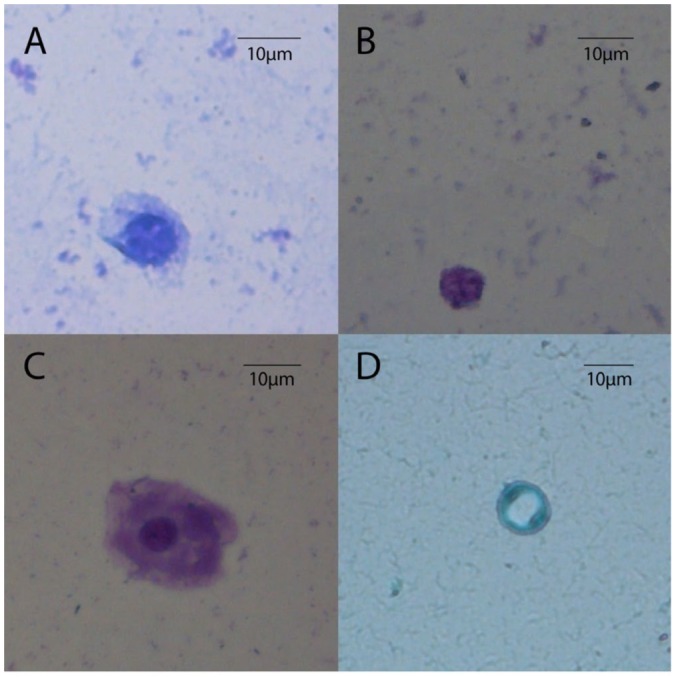
Protozoa found in PD effluent from CKD patients undergoing PD: A) Endolimax nana; B) Giardia sp.; C) Entamoeba sp.; and D) Blastocystis hominis. Images obtained after Giemsa stain observed at amplification of 400x. PD = peritoneal dialysis; CKD = chronic kidney disease.
Recent findings associated CKD to an impairment of the intestinal epithelial barrier structure and function that may enable translocation of microorganisms and other microbial products across the intestinal wall to systemic circulation (11,12). Peritoneal dialysis effluent protozoa colonization may represent a marker for the loss of intestinal epithelial barrier integrity in CKD patients. In the fecal samples of 3 PD patients, no intestinal protozoa were found by the research team or by the local laboratory. Since during the study, 2 protozoa-positive PD patients died, 1 with a peritonitis episode caused by Escherichia coli due to an intestinal perforation 5 months after sampling and the other due to sudden death, the collection of feces from these patients was not possible. The clinical history of the 5 PD patients colonized by protozoa, regarding signs and symptoms, peritonitis, and catheter exit-site (CES) infections revealed that 2 patients had an intestinal deregulation 6 months previous to effluent sampling and 1 patient had 2 peritonitis episodes by Streptococcus sp. and E. coli with intestinal perforation 1 and 5 months after sampling, respectively. Thus, our results could represent an accidental gut colonization acquired by water or food consumption. The absence of correlation with intestinal colonization was previously described in the literature (13), but it is important to note that the stools were collected after the PD effluent analysis and in some cases the time gap was superior to 6 months.
Entamoeba protozoa are recognized oral colonizers, although with low prevalence (14). Interestingly, this protozoon also colonized the saliva of the patient presenting Entamoeba sp. in PD effluent, suggesting that the digestive tract may represent in this case the source of this microorganism.
In order to understand the transmission routes of these microorganisms among the PD patients, it is crucial to characterize other environmental factors. In the group of PD patients presenting effluent protozoa colonization, clinical and environmental/social characterization was performed (Supplemental Table 1). In general, these 5 patients presented a low education level, different CKD etiologies, 1 being diabetic. Moreover, 2 out of 5 of these patients combined the following features: female, had contact with and frequently ate domestic poultry, water source was a private water-well, which made them more prone to contamination given their open access to the environment.
Despite the importance of these results, this study presented some limitations, such as the limited number of patients analyzed, and the applied methodology that presents lower sensitivity and specificity than other methods, such as enzyme immunoassays, polymerase chain reaction (PCR), as well as flow cytometry (15). Thus, one can hypothesize that with a more sensitive methodology, a higher prevalence of patients with PD effluent protozoa colonization could be detected. In conclusion, PD effluent of CKD patients undergoing PD may be susceptible to asymptomatic protozoa colonization. The clinical impact of this finding should be further investigated.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
LSS and ISS were supported by SFRH/BD/84837/2012 and SFRH/BPD/101016/2014 FCT/QREN–POPH/FSE. This work was financed by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2020 - Operacional Programme for Competitiveness and Internationalisation (POCI), Portugal 2020, and by Portuguese funds through FCT - Fundação para a Ciência e a Tecnologia/Ministério da Ciência, Tecnologia e Inovação in the framework of the project “Institute for Research and Innovation in Health Sciences” (POCI-01-0145-FEDER-007274); Study supported by Dental Medicine Faculty .
Footnotes
Supplemental material available at www.pdiconnect.com
REFERENCES
- 1. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3(5):1526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peces R, de la Torre M, Alcazar R. Visceral leishmaniasis and renal tuberculosis in a patient on maintenance haemodialysis. Nephrol Dial Transplant 1996; 11(4):707–8. [DOI] [PubMed] [Google Scholar]
- 3. Port FK, Held PJ, Nolph KD, Turenne MN, Wolfe RA. Risk of peritonitis and technique failure by CAPD connection technique: a national study. Kidney Int 1992; 42(4):967–74. [DOI] [PubMed] [Google Scholar]
- 4. Rocha A, Rodrigues A, Teixeira L, Carvalho MJ, Mendonca D, Cabrita A. Temporal trends in peritonitis rates, microbiology and outcomes: the major clinical complication of peritoneal dialysis. Blood Purif 2012; 33(4):284–91. [DOI] [PubMed] [Google Scholar]
- 5. Division of control of tropical diseases (CTD), progress report 1996. Geneva: World Health Organization (WHO). [Google Scholar]
- 6. Barazesh A, Fouladvand M, Tahmasebi R, Heydari A, Fallahi J. The prevalence of intestinal parasites in hemodialysis patients in Bushehr, Iran. Hemodial Int 2015; 19(3):447–51. [DOI] [PubMed] [Google Scholar]
- 7. Ohta M, Ikeda K, Miyakoshi H, Nishide K, Horigami T, Akao T, et al. A very rare case of continuous ambulatory peritoneal dialysis peritonitis caused by Anisakis larva. Am J Gastroenterol 1995; 90(10):1902–3. [PubMed] [Google Scholar]
- 8. Tilak R, Singh RG, Wani IA, Parekh A, Prakash J, Usha U. An unusual case of Acanthamoeba peritonitis in a malnourished patient on continuous ambulatory peritoneal dialysis (CAPD). J Infect Dev Ctries 2008; 2(2):146–8. [PubMed] [Google Scholar]
- 9. Boccardo G, De Prisco O, Ettari G, Donato G, Maurino D, Savoia D. Protozoan infection (Blastocystis hominis) concomitant with Pseudomonas sp. peritonitis in continuous ambulatory peritoneal dialysis (CAPD)]. Minerva Urol Nefrol 1996; 48(1):55–8. [PubMed] [Google Scholar]
- 10. Saxena R. Role of special histochemical stains in staining microorganisms. In: Dako , ed. Education Guide - Special Stains and H & E. 2nd ed. California: Dako; 2010: 99–107. [Google Scholar]
- 11. Vaziri ND. CKD impairs barrier function and alters microbial flora of the intestine: a major link to inflammation and uremic toxicity. Curr Opin Nephrol Hypertens 2012; 21(6):587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaziri ND, Yuan J, Norris K. Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 2013; 37(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeum CH, Ma SK, Kim SW, Kim NH, Kim J, Choi KC. Incidental detection of an Anisakis larva in continuous ambulatory peritoneal dialysis effluent. Nephrol Dial Transplant 2002; 17(8):1522–3. [DOI] [PubMed] [Google Scholar]
- 14. Ghabanchi J, Zibaei M, Afkar MD, Sarbazie AH. Prevalence of oral Entamoeba gingivalis and Trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, southern Iran. Indian J Dent Res 2010; 21(1):89–91. [DOI] [PubMed] [Google Scholar]
- 15. Barbosa J, Costa-de-Oliveira S, Rodrigues AG, Pina-Vaz C. Optimization of a flow cytometry protocol for detection and viability assessment of Giardia lamblia. Travel Med Infect Dis 2008; 6(4):234–9. [DOI] [PubMed] [Google Scholar]


