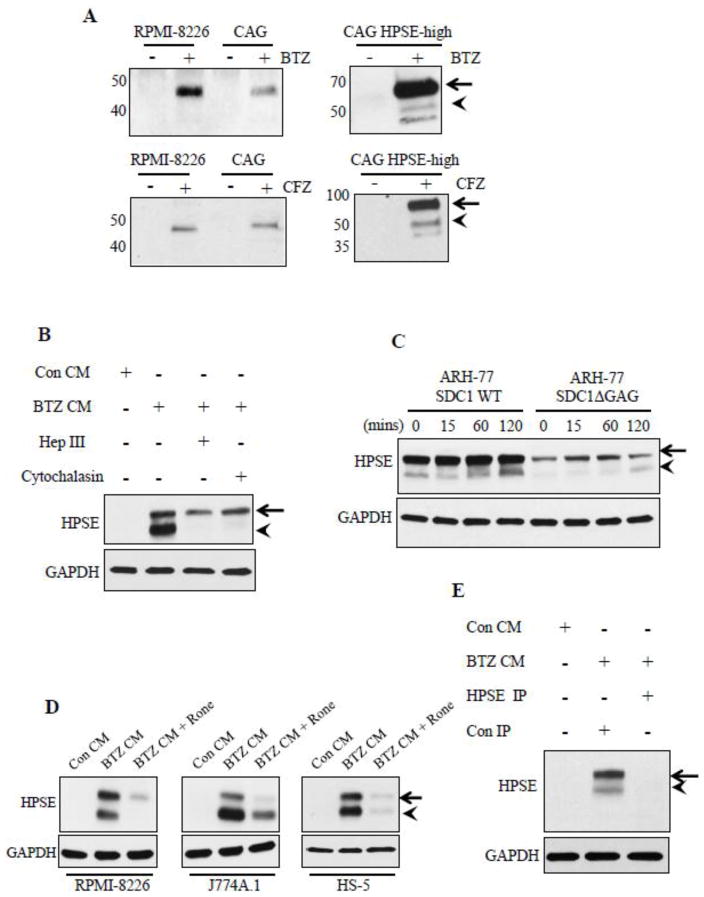
Figure 4.
Anti-myeloma therapy causes release of high levels of soluble heparanase from tumor cells. (A) Western blotting for HPSE in concentrated conditioned media from different myeloma cell lines after 24 h exposure to BTZ (0.5 μM) or CFZ (0.5 μM). (B) Western blotting for HPSE in RPMI-8226 cell extracts after 2 h incubation with concentrated conditioned media from BTZ treated HPSE-high cells (BTZ CM) or concentrated conditioned media from vehicle treated HPSE-high cells (Con CM). Controls included RPMI-8226 cells treated with heparinase III enzyme (10 μg/ml) or cytochalasin D (10 μg/ml) for 2 h prior to incubation with BTZ CM. (C) Blotting for HPSE in total cell extracts from ARH-77 cells expressing either full length human syndecan-1 (ARH-77 SDC-1 WT) or mutated syndecan-1 lacking glycosaminoglycan attachment sites (ARH-77 SDC1 Δ GAG) following their incubation with BTZ CM for 0–120 min. (D) RPMI-8226, J774A.1 cells (murine macrophage cells), and HS-5 (human bone marrow stromal cells) were treated with 6.75μM of Roneparstat (Rone) prior to incubation for 2 h with BTZ CM. Cells were extracted and probed for HPSE. Control included cells incubated with Con CM. (E) Western blot for HPSE in cell extracts from RPMI-8226 cells after treatment with Con CM, BTZ CM immunodepleted with Con IgG (Con IP) or with anti-HPSE antibody (HPSE IP). The absence of HPSE in RPMI-8226 cells incubated with HPSE immunodepleted BTZ CM confirms that HPSE detected in RPMI-8226 extracts is supplied by the BTZ CM.