Fig. 2.
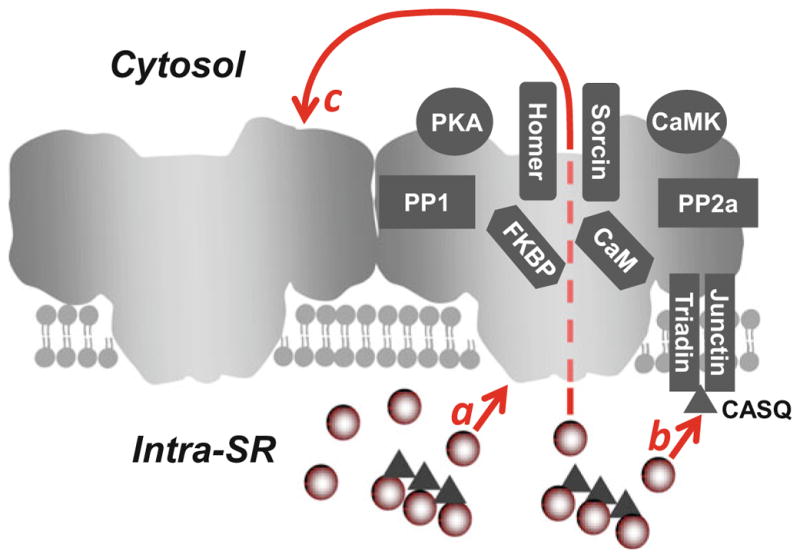
Regulation of cardiac ryanodine receptor. On the cytosolic side, RyR2 interacts with calmodulin (CaM), FK-506-binding proteins (FKBP), homer, sorcin, two major protein kinases (PKA and CaMKII), and two phosphatases (PP1 and PP2A). Luminal Ca2+ regulates RyR2 activity by directly binding to the luminal side of the channel (a). Moreover, triadin and junction form the luminal Ca2+ sensor via interactions with the SR Ca2+-buffering protein calsequestrin (CASQ; b). Luminal Ca2+ can also indirectly regulate RyR2 by acting on the cytosolic Ca2+ activation site of neighboring channels by a “feed-through” mechanism (c)
