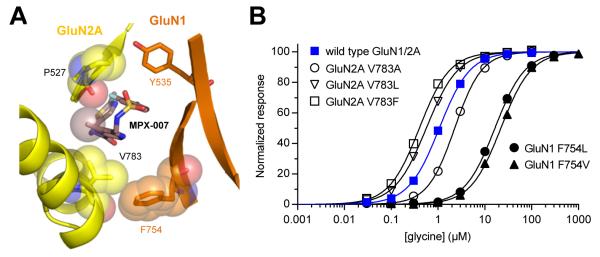
Figure 7. Mutational analyses of the influence of GluN2A V783 and GluN1 F754 on glycine potency.
A) View of the NAM binding site in the glycine/glutamate-bound GluN1/2A LBD heterodimer in complex with MPX-007. The van der Waals radius of the methyl group of MPX-007 is shown as transparent sphere. This methyl group contacts the backbone carbonyl of GluN2A P527 and the side chain of GluN2A V783. There is also a nonpolar interaction between side chains of GluN2A V783 and GluN1 F754.
B) Glycine concentration-response data for wild type and mutated GluN1/2A receptors. Responses are recorded in the presence of 100 μM glutamate using two-electrode voltage-clamp electrophysiology. Data are mean ± SEM from 9-16 oocytes.
See also Table S3.