Table 1.
Structure and inhibitory activity of 2-(pyridin-3-yl)quinazoline derivatives with substituted cyclohexyl and related ringsa
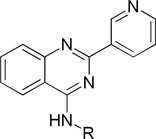 | |||||
|---|---|---|---|---|---|
| Comp. | R | IC50 (µM) | Comp. | R | IC50 (µM) |
| 4 | 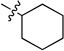 |
0.177 ± 0.012 | 6f | 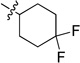 |
0.234 ± 0.017 |
| 6a | 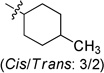 |
0.056 ± 0.005 | 6g | 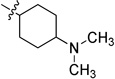 |
Inactive |
| 6b | 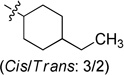 |
0.126 ± 0.007 | 6h | 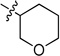 |
0.891 ± 0.023 |
| 6c | 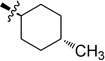 |
0.020 ± 0.002 | 6i | 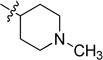 |
Inactive |
| 6d | 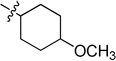 |
0.251 ± 0.018 | 6j | 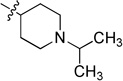 |
33.21 ± 9.71 |
| 6e | 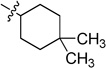 |
0.431 ± 0.042 | 6k | 36.07 ± 8.72 | |
Experiments were performed in triplicate, and the mean ± SD is shown.
