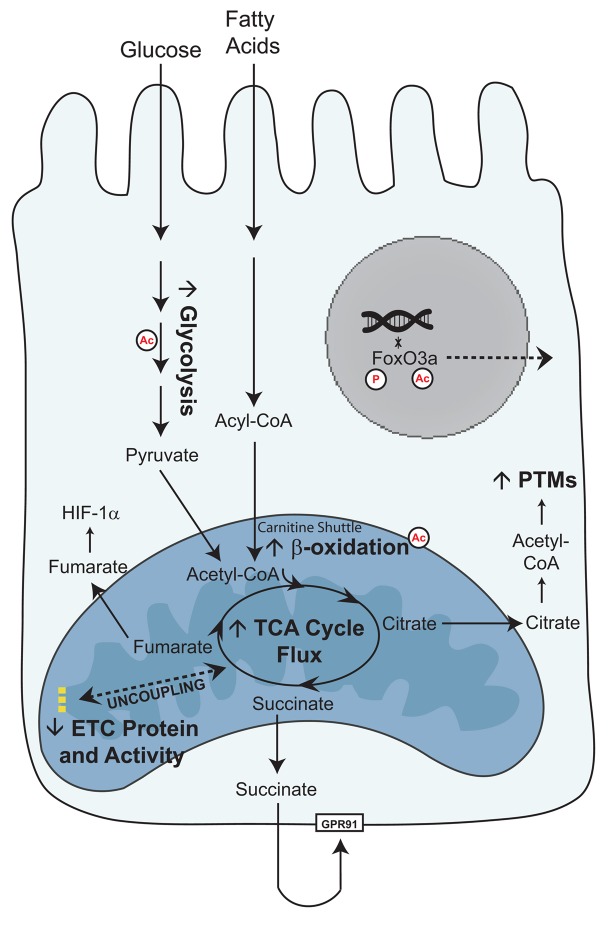
Figure 9. Schema of glucose and fatty acid metabolism in the diabetic proximal tubule cell.
As the glucose concentration in plasma or urine increases, more glucose is transported into kidney proximal tubule cells. Glycolysis increases, resulting in increased pyruvate levels and TCA cycle activity in the mitochondria. Fatty acids are broken down into acyl-CoAs and transported across the mitochondrial membrane as acylcarnitines. β-Oxidation increases, resulting in increased TCA cycle activity. Although mitochondrial metabolism is elevated, there is a concurrent lack of increased ATP production through the electron transport chain (26). We propose that an uncoupling between mitochondrial metabolism and oxidative phosphorylation may underlie the metabolic phenotype of diabetic kidney disease. The increased TCA cycle metabolites can perturb normal cellular function. High levels of citrate can be used as a substrate for post-translational modifications (PTMs), such as acetylation (Ac), which can alter the activity of metabolic enzymes and localization of transcription factors. Succinate, through GPR91, can activate the renin-angiotensin system, and fumarate can induce HIF-1α. These perturbations can promote progression of DKD.