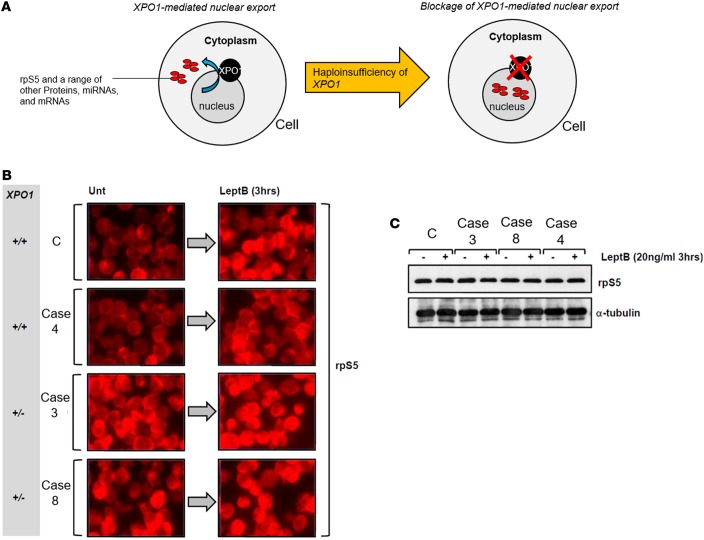
Figure 4. XPO1 dysfunction in patients’ cells as determined by abnormal distribution of rpS5.
(A) Normal physiological XPO1-mediated export of 40S rpS5 and approximately 200 different proteins, mRNAs, and miRs from the nucleus to the cytoplasm. Suppression of XPO1 leads to blocked XPO1-mediated cargo and retention of proteins, mRNAs, and miRs in the nucleus. (B) Immunofluorescence images (original magnification, ×40) of patients’ LCLs showing that haploinsufficiency of XPO1 was associated with impaired nuclear export of a known cargo protein, rpS5. LCLs were either untreated (Unt) or treated with leptomycin B (LeptB), which inhibits XPO1-mediated nuclear export of cargo. Untreated control LCLs (C) showed a preponderance of cytoplasmic rpS5 staining, consistent with its role at the ribosome. Upon leptomycin B treatment, nuclear accumulation of rpS5 was evident, consistent with XPO1 inhibition. A similar response was evident in LCLs from the patient in case 4, who possessed 2 copies (+/+) of XPO1. In stark contrast, LCLs from individuals with a deletion of XPO1 (+/–) (cases 3 and 8) each exhibited significant nuclear accumulation of rpS5 when untreated, and this distribution was unaffected by treatment with leptomycin B. (C) Western blotting of WCEs from LCLs untreated (–) or treated (+) with leptomycin B showed equal rpS5 expression. LCLs, lymphoblast cell lines; WCEs, whole-cell extracts.