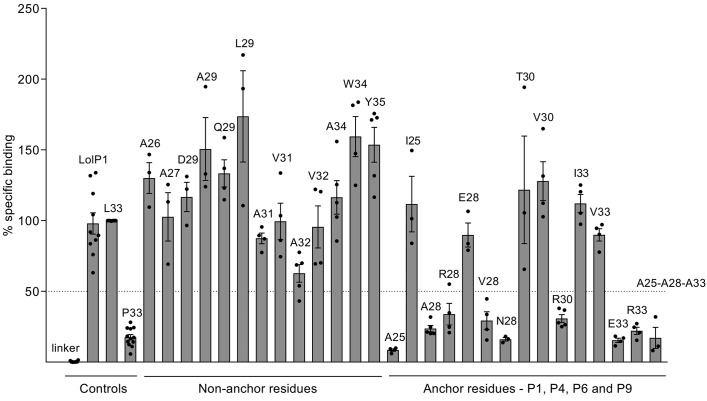
Figure 3. Peptide binding to the DRA/DRB3ai*01:01-positive cell line STEINLIN.
Peptides (12-mers) with single or multiple amino acid substitutions compared with HPA-1a peptide, and extended with a biotinylated linker peptide, were incubated with STEINLIN cells at 5 μM and 10 μM peptide in the presence of 2.5 μM AdEtOH. The efficiency of binding to APCs was assessed by flow cytometry with streptavidin-conjugated R-PE. Binding was measured as percent specific binding (L33 peptide defined as 100%). Each peptide that bound efficiently to STEINLIN was also examined for binding to MHC-matched cell lines DUCAF, EMJ, and EK such that binding to any other MHC class II molecule on STEINLIN could be ruled out. Data points from independent experiments are presented as dots, with bars representing mean ± SEM of at least 3 experiments. Raw data values were median R-PE fluorescence intensity on B-LCLs (gated by light scatter cytogram). Background intensity (cells only, no peptide) was subtracted before calculating the specific binding within each experiment. AdEtOH, adamantane ethanol; APC, antigen-presenting cell; R-PE, R-phycoerythrin; B-LCL, B-lymphoblastoid cell lines