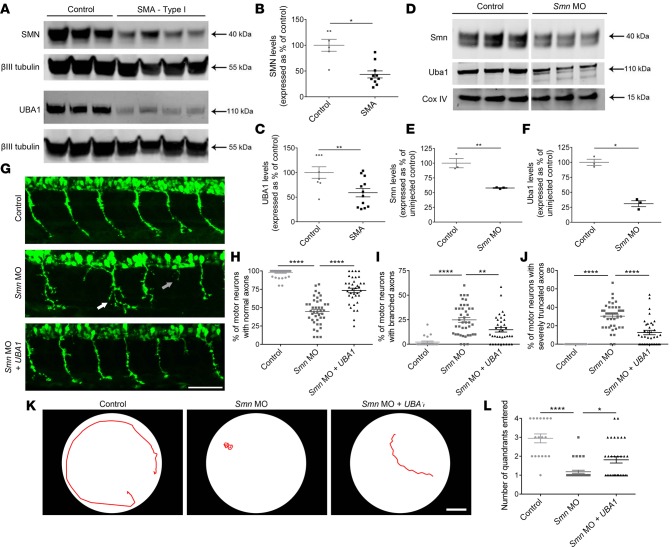
Figure 1. UBA1 loss in patient-derived iPSC motor neurons and SMA zebrafish, with rescue of zebrafish motor pathology following Uba1 restoration.
(A–C) Significant reduction of survival motor neuron (SMN) and ubiquitin-like modifier activating enzyme 1 (UBA1) protein in type I spinal muscular atrophy (SMA) patient iPSC-derived motor neurons, as quantified by Western blot (independent clones per genotype: control n = 9 and SMA n = 8; unpaired, 2-tailed Student’s t test). (D–F) Significant reduction in Smn and Uba1 protein levels in zebrafish injected with 6 ng morpholino oligonucleotide (MO) targeted against Smn compared to uninjected controls, as quantified Western blot analysis (n = 3, batches of 30 fish per lane; unpaired, 2-tailed Student’s t test). Lanes were run on the same gel but were noncontiguous. (G) Representative micrographs of spinal motor axons from uninjected control zebrafish, zebrafish injected with 4 ng Smn MO (white arrow indicates abnormal axon branching, gray arrow indicates severely truncated axons), and zebrafish injected with 4 ng Smn MO coinjected 200 ng/μl human UBA1 mRNA at 30 hours after fertilization (scale bar: 50 μm). (H–J) Significant improvement in the percentage of normal, branched, and severely truncated motor axons in zebrafish injected with 4 ng Smn MO coinjected 200 ng/μl human UBA1 mRNA (n = 20 per treatment group; 1-way ANOVA with Tukey’s post-hoc test). (K) Representative tracings of automated swim path analysis of uninjected control zebrafish, zebrafish injected with 4 ng Smn MO, and zebrafish injected with 4 ng Smn MO coinjected 200 ng/μl human UBA1 mRNA at 3 days after fertilization (scale bar: 1 cm). (L) Significant improvement in the number of quadrants entered during automated swim path analysis of zebrafish injected with 4 ng Smn MO coinjected 200 ng/μl human UBA1 mRNA (control n = 18, Smn MO n = 33, Smn MO + UBA1 n = 32; Kruskal-Wallis test with Dunn’s post-hoc test). *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.001.