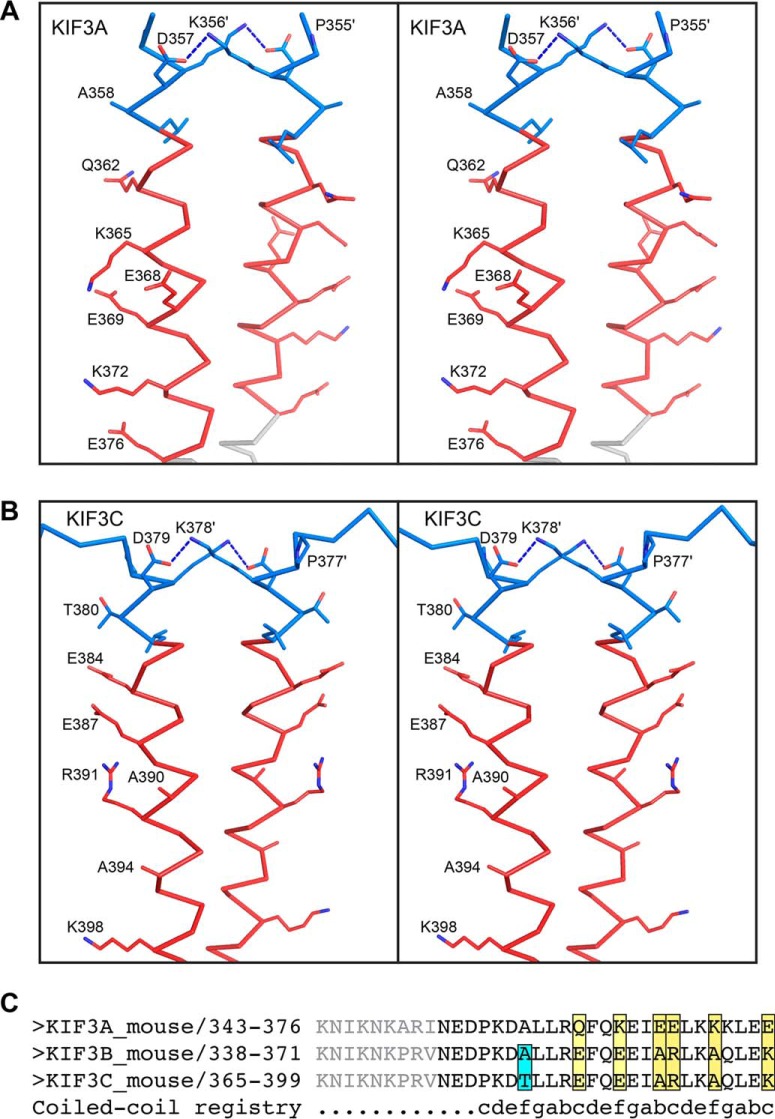
FIGURE 4.
Selected side-chain interactions in and the sequence differences between KIF3A and KIF3C neck-linkers shown in stereo. KIF3A and KIF3C are colored as in Fig. 2, with side chains shown as sticks and colored by element. In both KIF3A (A) and KIF3C (B), there is a hydrogen bonding interaction between a lysine and an aspartate that stabilizes part of the coiled-coil. The sequences of the neck-linker and α7 are also shown (C) where the residues depicted in gray were not included in the constructs but represent the full-length linker. Interestingly, none of the differences in sequence between KIF3A and KIF3C are predicted to influence formation of a heterodimer.