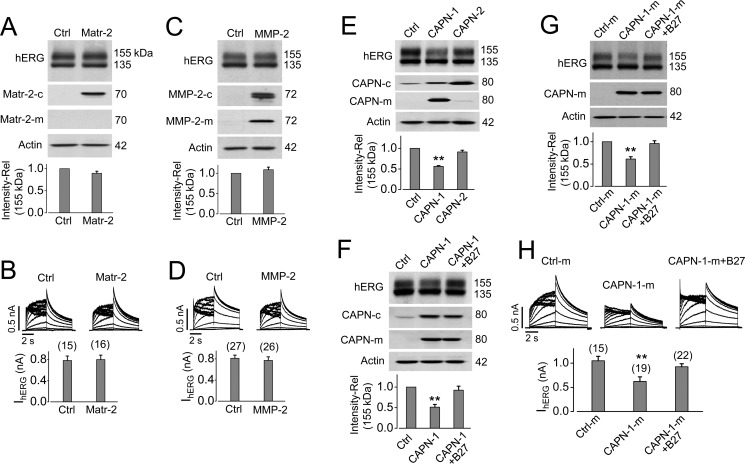
FIGURE 9.
Extracellular calpain-1 decreases mature hERG expression and IhERG. A and B, Matriptase-2 does not affect hERG expression or IhERG. hERG-HEK cells were transfected with pcDNA3 (control (Ctrl)) or matriptase-2 (Matr-2). Expression of hERG and matriptase-2 from whole-cell lysate (Matr-2-c), and matriptase-2 from culture medium (Matr-2-m) are shown in A, whereas IhERG from cells transfected with pcDNA3 (control) or matriptase-2 plasmid are shown in B. C and D, MMP-2 does not affect hERG expression or IhERG. Expression of hERG and MMP-2 from whole-cell lysate (MMP-2-c) and MMP-2 from culture medium (MMP-2-m) are shown in C, whereas IhERG from cells transfected with pcDNA3 (control) or MMP-2 plasmid is shown in D. E, calpain-1 (CAPN-1), but not calpain-2 (CAPN-2), decreases mature hERG expression. Expression of hERG, CAPN-1, or CAPN-2 from whole-cell lysate (CAPN-c) and CAPN-1 and CAPN-2 from culture medium (CAPN-m) are shown. CAPN-1 but not CAPN-2 transfection led to its elevated expression in culture medium, which was associated with a decrease in the 155-kDa hERG band. F, cell membrane-impermeant calpain inhibitor peptide B27 (1 μm) abolishes CAPN-1-mediated hERG reduction. G and H, the culture medium of calpain-1-transfected cells decreases mature hERG expression and IhERG, and these effects are abolished by the calpain inhibitor peptide B27. For Western blotting analysis of hERG expression in A, C, E, F, and G, the intensities of the 155 kDa band are normalized to the control and expressed as relative values (n = 3–7). Actin was used as a loading control. For IhERG analyses in B, D, and H, tail current amplitudes under each treatment are summarized below representative current traces. The numbers above the bar graphs indicate the number of cells examined from 3–5 independent experiments. **, p < 0.01 versus control. Error bars, S.E.