FIGURE 6.
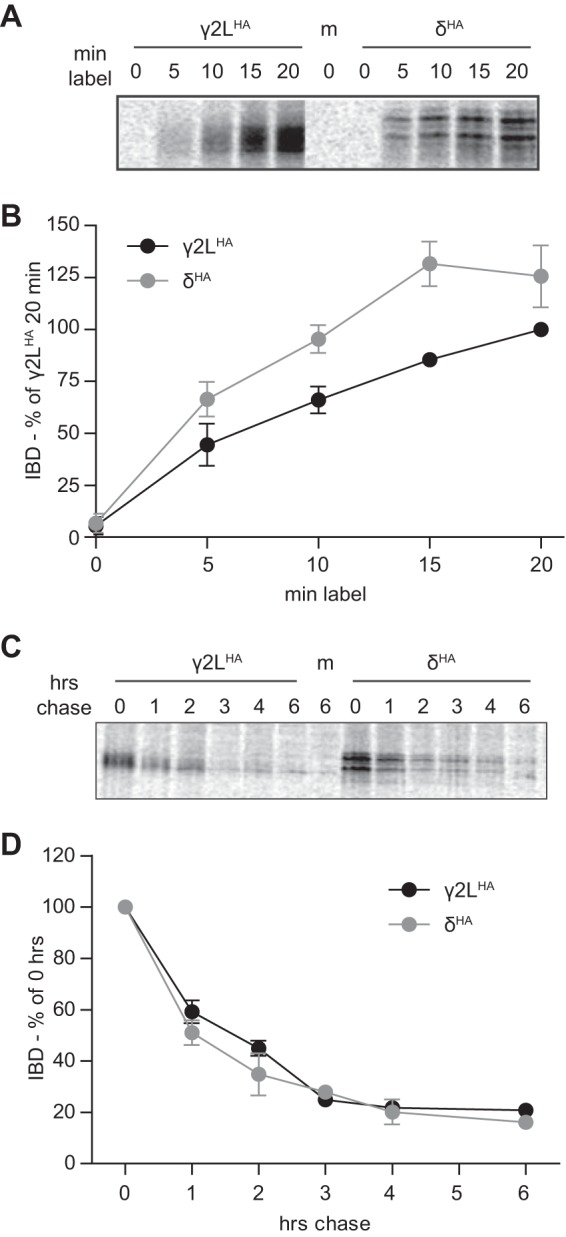
GABAA receptor γ2LHA and δHA subunits were synthesized at similar rates, and when newly synthesized, were degraded at similar rates. Metabolic labeling was used to assess the synthesis and degradation rates of γ2LHA and δHA subunits. A and B, HEK293T cells expressing equivalent amounts of γ2LHA or δHA subunits were cultured for 0–20 min in media containing [35S]methionine. Subunit protein was isolated from cell lysates by immunoprecipitation and separated by SDS-PAGE. A presents a representative gel exposure; B presents a quantification of band intensity (IDV) averaged from four separate experiments. Band intensities were normalized to those of the 20-min incubation condition. C, HEK293T cells expressing equivalent amounts of γ2LHA or δHA subunits were cultured for 20 min in media containing [35S]methionine. To assess degradation rates of this newly synthesized protein population, radioactive media were subsequently replaced by regular media, and cells were returned to incubators for 0–6 h. Subunit protein was isolated from cell lysates by immunoprecipitation and separated by SDS-PAGE. C presents a representative gel exposure, and D presents a quantification of band intensity (IDV) averaged from four separate experiments. Band intensities are normalized to that of the 0-min chase condition.
