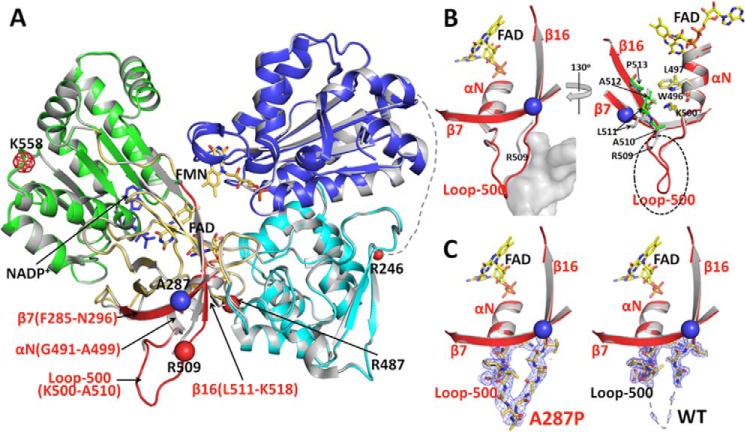
FIGURE 7.
Comparison of the crystal structures of WT and A287P POR. A, overlay of the structures of A287P mutant and wild type POR. The superposition was done using the entire 603 visible residues. Wild type POR is colored gray, and the A287P structure is colored by domains as follows: the FMN-binding domain (blue), connecting domain (cyan), FAD-binding domain (gold and red), NADP(H)-binding domain (green), and the hinge between the two flavin-binding domains is shown as a dotted line with its last residue Arg-246 marked as a small red ball. The mutation site, A287P, is shown with a blue ball. The region in the FAD-binding domain showing minor but significant structural changes caused by the A287P mutation is shown in red. Following the nomenclature used for the structure of rat POR (12), secondary structural elements in this region are listed in red. β7 (Phe-285–Asn-296) is antiparallel to β16 (Leu-511–Lys-518), and part of these two β-strands are packed onto the αN-helix (Gly-491–Ala-499). The red balls are possible trypsin digestion sites consistent with the results of both limited trypsinolysis experiments and the mass spectrometry (MS) analyses of trypsinized fragments, except that the meshed red ball (Lys-558) site has not been confirmed by the MS analysis. See under “Results” for the relationship between possible trypsin cleavage sites and the cleavage pattern. B, two enlarged views of the vicinity of the mutation site in the structure of A287P (the red region shown in A). Left panel, a similar view to A. In the mutant structure, the A287P mutation causes a slight bending of the N-terminal side of β7, which pushes the neighboring β16 and part of the connected loop-500 outward. Although these changes are small (the distance between Cα carbons of Pro-287 of the mutant and Ala-287 of WT is 1.6 Å, and the distance between two Arg-509 Cα carbons is ∼2 Å), the loop-500 in A287P was then stabilized by a neighboring molecule (gray mass shown in the left panel, molecule B) in the crystal packing, making loop-500 of molecule A in A287P visible in the crystal structure. It is possible that, without the crystal packing restriction in solution, Loop-500 and part of β16 can be pushed out further, so that residues in β16 in the A287P mutant make closer contact with Trp-496, Leu-497, and Lys-500 in helix αN (Gly-491–Ala-499). Thus, the mutation at Ala-287 to proline can cause a disturbance of the αN-helix, which propagates to the entire FAD domain of the mutant molecule, resulting in the unstable A287P protein. Right panel, view with ∼130° rotation from the left panel view. For clarity, the part of the neighboring molecule (molecule B) covering Loop-500 (the gray cloud represented in the left panel) is not shown in this view. In the mutant structure, the A287P mutation causes a slight bending of the N-terminal side of β7, which pushes the neighboring β16 and part of the connected Loop-500 outward. Although these changes are small, the root mean square distance between Cα carbons of A287P and WT for this part of β16 (residues Arg-509–Pro-513) is 1.9 Å (corresponding value for 603 residues of the entire molecule is 0.3 Å). Loop-500 in A287P is then stabilized by a neighboring molecule (molecule B) in the crystal packing, resulting in Loop-500 of molecule A in the A287P structure visible in the mutant structure. It is certainly possible that, in solution without the crystal packing restriction, Loop-500 and part of β16 can be pushed further to make close contact with residues Trp-496, Leu-497, and Lys-500 in helix-αN (Gly-491–Ala-499), thus separating the two subdomains, FAD-binding and NADPH-binding domains, and making the mutant protein unstable and more susceptible to proteolysis (see Figs. 6A and Fig. 7A). C, electron density maps for Loop-500. Comparison of 2Fo − Fc electron density maps contoured at 1σ level (gray mesh) corresponding to Loop-500 in the mutant (left panel) and wild type structures (right panel).