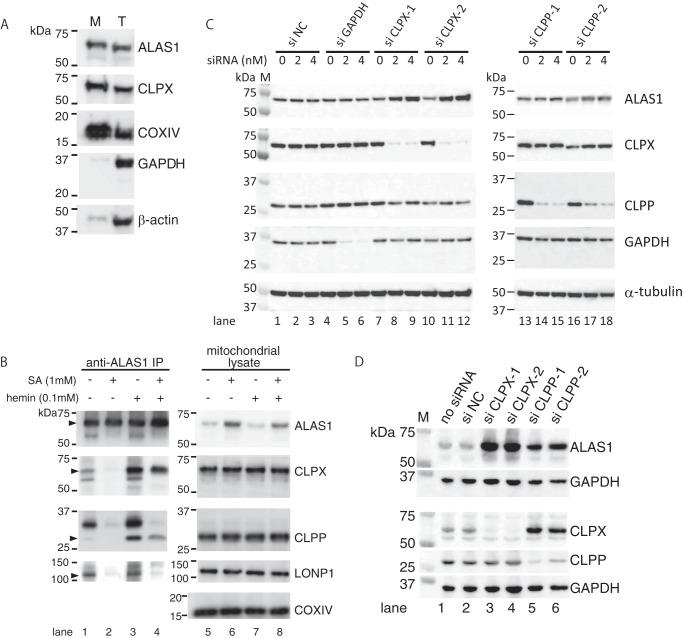
FIGURE 3.
Endogenous ALAS1 forms a complex with ClpXP in a heme-dependent manner in mitochondria, and the specific suppression of CLPX or CLPP results in the accumulation of ALAS1 proteins within HepG2 cells. A, the purity of the mitochondria in the mitochondria-rich fraction was determined by detecting mitochondria-specific proteins (ALAS1, CLPX, and COXIV), cytosolic protein (GAPDH), or cytoskeleton protein (β-actin) using Western blotting analysis. B, HepG2 cells were incubated with (lanes 2, 4, 6, and 8) or without (lanes 1, 3, 5, and 7) SA for 24 h. Some cell samples were subsequently treated with hemin for 30 min (lanes 3, 4, 7, and 8). Endogenous ALAS1 protein was immunoprecipitated using an anti-ALAS1 monoclonal antibody, and the loading volume of each eluate was adjusted according to the intensity of each immunoprecipitated ALAS1 protein; therefore, each sample contained a similar amount of ALAS1 protein (lanes 1–4). For total cell lysate, 10 μg of protein was loaded into each lane (lanes 5–8). C, specific siRNA-mediated reduction in CLPX expression causes accumulation of ALAS1 proteins in HepG2 cells. The transfected siRNAs and their concentrations are indicated at the top of the panel. Total cell lysate was prepared for each cell sample, and 10 μg of protein was loaded into each lane. D, prolonged suppression of CLPP or CLPX induced the accumulation of endogenous ALAS1 protein in HepG2 cells. The cells were harvested 10 days after the initial transfection of each siRNA. The details of this procedure are described under “Experimental Procedures.” A total of 10 μg of protein was loaded into each lane.