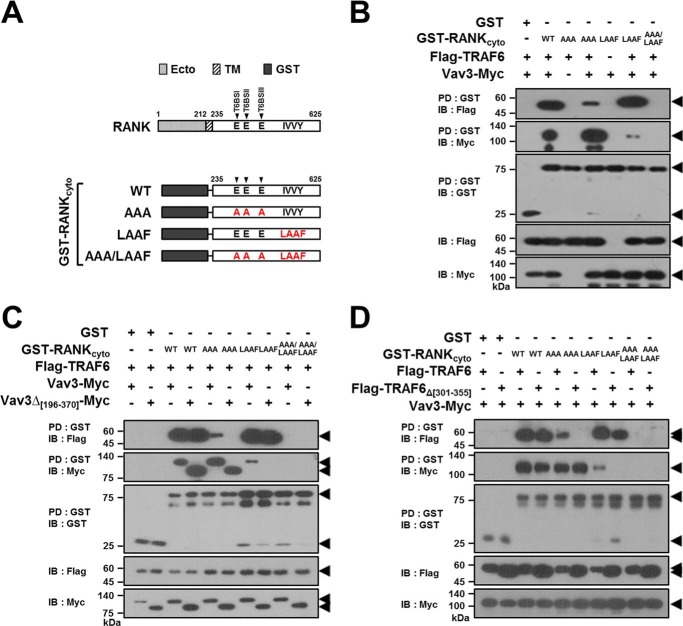
FIGURE 7.
TRAF6-Vav3 interaction forms a receptor complex with RANKcyto lacking either T6BSs or the IVVY motif. A, schematic diagram of GST-fused RANKcyto mutants. The extracellular domain (Ecto) and transmembrane domain (TM) in RANK are indicated. The numbers shown above each domain diagram denote the amino acid numbers. The Ala mutations of the Glu residues for the T6BS consensus sequences (PXEXX[Ar/Ac]) and the LAAF mutation for the IVVY motif are indicated in RANKcyto. The arrow indicates the position of T6BSs. B, signaling complex formation by the TRAF6-Vav3 interaction in RANKcyto lacking either T6BSs or the IVVY motif. 293T cells were transfected with GST-RANKcyto (1.0 μg), Vav3-Myc (1.0 μg), and FLAG-TRAF6 (1.0 μg). The cell lysates were normalized for total protein content. GST-RANKcyto was pulled down (PD) using GST beads, and the PD GST-RANKcyto was visualized via anti-GST (B-14) immunoblotting (IB, third panel). The proteins bound to the GST-RANKcyto were visualized via anti-FLAG (M2) immunoblotting (top panel) or anti-Myc (9E10) IB (second panel). The level of TRAF6 or Vav3 expression was detected via anti-FLAG immunoblotting (fourth panel) or anti-Myc IB (bottom panel). C, the effect of the deletion of the DH domain of Vav3 on signaling complex formation by the RANKcyto lacking either T6BSs or the IVVY motif. D, the effect of the deletion of the CC domain of TRAF6 on signaling complex formation by the RANKcyto lacking either T6BSs or the IVVY motif. All experiments were performed at least three times with similar results.