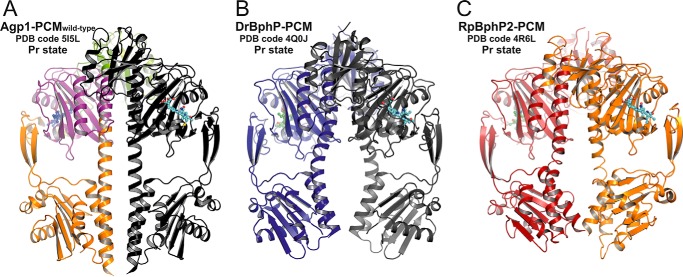
FIGURE 3.
Comparison of parallel dimer structures in prototypical phytochromes in the Pr state. A, crystallographic dimer of wild-type Agp1-PCM is shown in ribbon representation (PDB code 5I5L; same color code as in Fig. 2C). B, crystallographic dimer of DrBphP-PCM (PDB code 4Q0J) is shown, with one monomer in dark blue and the symmetry-related monomer in gray. C, non-crystallographic dimer of RpBphP2-PCM (PDB code 4R6L) is shown, with one monomer in firebrick-red and the second in orange. In all three panels the chromophore biliverdin is depicted as balls and sticks, and the structures are shown in identical views after superpositioning of the PAS-GAF bidomains. The dimer structures are similar in the regions defined by the PAS and GAF domains only. Because of the different helical spines and in particular the different bending of the long GAF-PHY helices, however, the size of the gaps between the PHY domains along the symmetry axes and the relative orientations of the C-terminal helices leading to the His kinase modules are significantly different between the three structures.