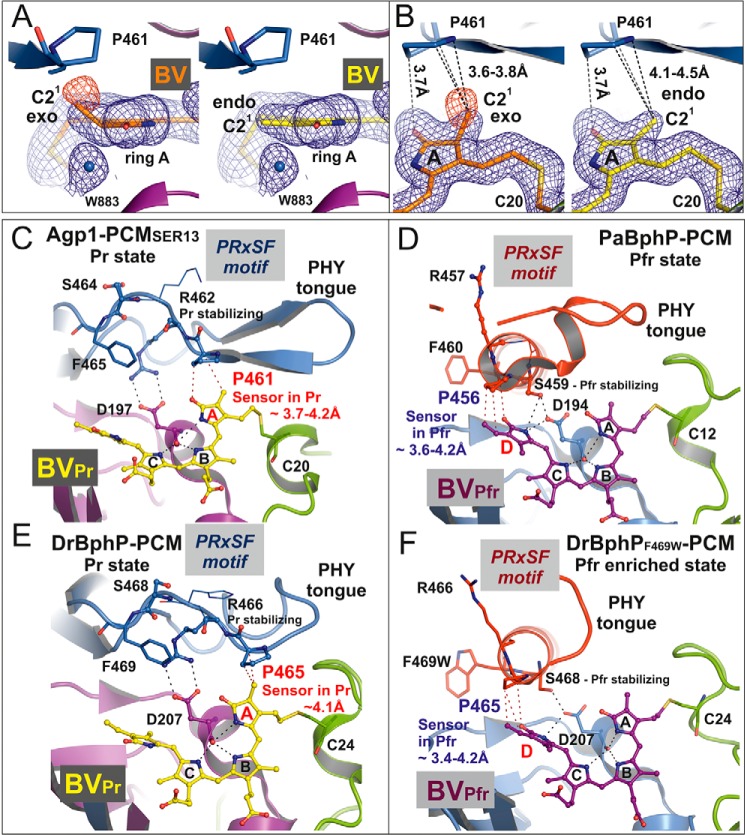
FIGURE 6.
Structure of the biliverdin chromophore at pyrrole ring A and the PRXSF motif as directly interacting signal transducer module in bacterial phytochromes. A, electron density of ring A of the chromophore BV after, left panel, refinement with geometric restraints for a PΦB-like structure with an exocyclic C3=C31 double bond or, right panel, with restraints for a BV-like structure with an endocyclic C2=C3 double bond in ring A. The blue mesh represents a weighted 2Fo − Fc map contoured at 1.0 σ. That the PΦB-like chromophore structure is wrong is indicated by the negative electron density at the C21 methyl group, as shown by the red mesh that represents the weighted Fo − Fc map at −2.0 σ. B, similar representation as in A in a different orientation. In both panels the shortest distances between Pro-461 of the PRXSF motif and the C21 atom in the PΦB-like structure with an exocyclic double bond, left panel, and in the BV-like structure with an endocyclic double bond, right panel, are indicated by dashed lines. C–F, chromophore binding pockets in the crystal structures of different bacteriophytochromes are shown with BV, the highly conserved Asp of the conserved PXXDIP motif in the GAF domain, and the amino acids of PRXSF motif as balls and sticks or thin lines (x and Phe of the PRXSF motif). The polypeptide chains are depicted in ribbon representation. C and E, in the crystal structures of Agp1-PCM-SER13 (PDB code 5I5L) and DrBphP-PCM (PDB code 4Q0J), respectively, in their Pr states, the tongue of the PHY domain is folded as a β-hairpin loop over the chromophore binding pocket. In the Pr structure of Agp1-PCM-SER13, Pro-461 of the PRXSF motif in the tongue region (as well as Pro-465 in the Pr state of DrBphP-PCM) undergoes van der Waals interactions with ring A of the chromophore BV, thereby being poised to act as sensor of structural changes that occur at ring A after photoactivation of the Pr state. Arg-462 (Arg-466 in DrBphP) stabilizes Asp-197 (Asp-207 in DrBphP) via a salt bridge. Ser-464 (Ser-468 in DrBphP) is solvent-exposed and without any interaction with the chromophore region in the Pr state crystal structures. D and F, in the crystal structures of PaBphP-PCM (PDB code 3NHQ) and DrBphP-PCM (PDB code 5C5K) in their Pfr (D) or Pfr-enriched (F) states, respectively, the tongue of the PHY domain is folded as α-helical and loop structure over the chromophore binding pocket. Pro-456 of the PRXSF motif in PaBphP-PCM (or Pro-465 in the Pfr-enriched state of DrBphP-PCM) is in van der Waals contact with ring D of the chromophore BV, in a position to sense structural changes that occur at ring D after photoactivation of the Pfr state. Ser-459 (Ser-468 in DrBphP) stabilizes Asp-194 (Asp-207 in DrBphP) of the conserved PXXDIP motif via a hydrogen bond. Arg-457 in PaBphP and the corresponding residue Arg-466 in DrBphP are solvent-exposed and without any interactions with the chromophore regions in the crystal structures of the Pfr and the Pfr-enriched state, respectively. Parts of the secondary structures in front of the chromophore binding pocket were removed for clarity in C–F.