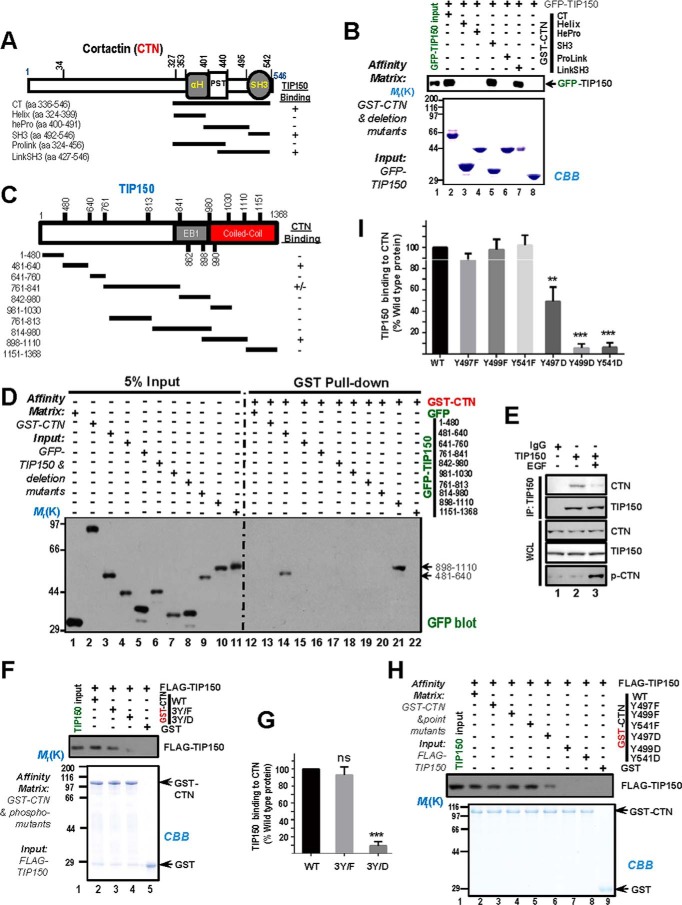
FIGURE 3.
Phosphorylation of CTN in its SH3 domain regulates the CTN-TIP150 interaction. A, schematic of deletion mutants of CTN C-terminal. +, positive; −, negative. aa, amino acids. B, purified GST-CTN and its binding domains (CT, C-terminal; Helix, helical; hePro, helical proline-rich; SH3; ProLink, proline-rich; and LinkSH3) fragments were used to absorb GFP-TIP150 from the HEK293T cell lysates. GST-CTN and its bound materials were resolved by SDS-PAGE followed by Coomassie Brilliant Blue (CBB) staining and immunoblotted with an anti-GFP antibody. C, schematic of TIP150 deletion mutants. +, positive; −, negative. D, purified GST-CTN was used as an affinity matrix to isolate proline-rich mutants of GFP-TIP150 from the lysates of HEK293T cells. Western blotting was performed as described in B. E, serum-starved MDA-MB-231 cells were treated with or without EGF. Endogenous TIP150 was immunoprecipitated, and precipitated endogenous CTN was detected by Western blotting. IP, immunoprecipitation. WCL, whole cell lysate. F, phospho-mutants of purified GST-CTN at tyrosine sites 421, 466, and 482 were used as affinity matrices to isolate FLAG-TIP150 from the lysates of HEK293T cells. Western blotting was performed as described previously. G, the intensity of bands as shown in F was quantified and normalized to that of CTNWT. Data represent mean ± S.E. from three independent experiments. ***, p < 0.001; ns, not significant. H, phospho-mutants of purified GST-CTN were used to absorb FLAG-TIP150 from the HEK293T cell lysates. After washing, proteins bound to agarose beads were analyzed by Coomassie Brilliant Blue staining and Western blotting using an anti-FLAG antibody. I, the intensity of bands as shown in H was quantified and normalized to that of CTNWT. Data represent mean ± S.E. from three independent experiments. **, p < 0.01; ***, p < 0.001.