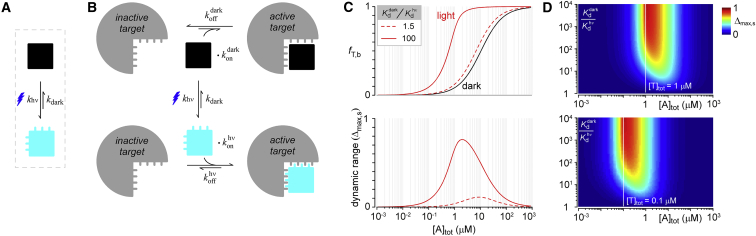
Figure 2.
Schematic depiction of the behavior of an optogenetic actuator. (A) Light-activation leads to conformational changes in the actuator. (B) Four molecular conformations are possible: unbound dark actuator, bound dark actuator, unbound lit actuator, and bound lit actuator. Physical changes resulting from light allow the protein to interact better with its target under illumination and bias it to reside as a bound lit actuator. (C) The top graph shows a simulation for the changes in fraction bound of actuator-target complexes as a function of actuator concentration due to different fold changes in actuator binding affinity before and after light. Similarly, the bottom graph illustrates the dynamic range of actuator-target complexes for these various conditions. (D) Top graph color maps dynamic range at a total target concentration of 1 μM as a function of total actuator concentration and binding affinity fold enhancement for lit versus dark states. An analogous graph is drawn on the bottom for a total target concentration 0.1 μM. (Open line) Total concentration of actuator and target are equal, which is also the optimal total actuator concentration for maximizing dynamic range. To see this figure in color, go online.