Abstract
Among currently available technologies, transcranial direct current stimulation (tDCS) is one of the most promising neuroenhancements because it is relatively effective, safe, and affordable. Recently, lay people have begun to build—or purchase—the tDCS device to use it at home for treatment or as a cognitive enhancer. The tDCS device is currently not covered by the existing regulatory framework, but there are still significant potential risks of misusing this device, and its long-term effects on the brain have not been fully explored. Thus, researchers have argued the need for regulations or official guidelines for the personal use of tDCS. However, until now, no systematic research on the do-it-yourself (DIY) tDCS user community has been done. The present study explores the basic demographic characteristics of DIY tDCS users as well as why and how they are using this device through a questionnaire survey, in-depth interviews, and a content analysis of web postings on the use of tDCS. This preliminary but valuable picture of the DIY tDCS user community will shed light on future studies and policy analysis to craft sound regulations and official guidelines for the use of tDCS.
Keywords: cognitive enhancement devices, do-it-yourself (DIY) tDCS, FDA regulation, transcranial direct current stimulation (tDCS)
INTRODUCTION
Human beings’ efforts to enhance cognitive ability have a long history. Language can be counted as the first cognitive enhancement, and the invention of writing also ‘dramatically improved our cognitive ability to remember, to learn, and to communicate’.1 Today, with the aid of recent phenomenal development of biomedical technology, we are witnessing the emergence of more direct biological cognitive enhancements—‘chemical, physical, or electromagnetic intrusions into our physical brain’.2
Brain enhancement has been considered as one of the most important intersections of law and neuroscience.3 The idea of directly enhancing the brain with drugs, brain stimulation, or neurosurgery might seem frightening. But more than frightening, the use of these enhancement technologies raises crucial neuroethical and legal issues.4 For example, fairness can be a serious problem, if an effective enhancement technology is very costly and thus, available only to the rich. Also, individuals could be coerced, explicitly or implicitly, to undergo cognitive enhancements for various reasons, such as criminal rehabilitation, medical treatment, or even academic success at school.
Despite these potential risks, Professor Hank Greely has argued that safe and effective cognitive enhancement will benefit both individuals and society, and we should ‘require better research on, and better regulation of, cognitive enhancement’ instead of banning the use of all cognitive enhancements:
Biomedicine will be creating more and more products and procedures that can be used for cognitive enhancement. Some of them will be used in ways that will, on balance, improve human life and society. At the same time I worry that they may be used in harmful ways. I am confident, though, that a knee-jerk rejection of all direct brain enhancement will be at least a missed opportunity and at worst an opening for a damaging underground and uncontrolled world of enhancement. In order to maximize the benefits and minimize the harms of these new technologies, we will need to look at particular enhancements rationally and to adopt, ban or regulate them carefully.5
One of the most eye-catching and readily accessible direct brain enhancement technologies is transcranial direct current stimulation (tDCS). tDCS is a type of non-invasive neuromodulation, which delivers weak direct current (DC) (1–2 mA) to the brain using small saline-soaked electrodes. This weak current is insufficient to cause neurons to fire, but it can ‘alter the excitability of neurons by shifting their membrane potentials in a de- or hyper polarizing direction; thus making them more or less likely to fire’.6 Anodal DC stimulation is known to increase neuronal excitability, while cathodal stimulation decreases it.7
The history of tDCS and the current popularity of tDCS as a cognitive enhancement
Application of electric currents to the human brain for therapeutic purposes is not a new idea. In ancient times, people placed an electric fish over the scalp to treat headache or epilepsy. Later in 18th century, advances in the science of electrophysiology by Galvani and Volta inspired the use of direct currents for the treatment of mental disorders.8 Nonetheless, erratic results and the advent of electroconvulsive therapy led to the decrease of interest in DC brain stimulation.9
However, reappraisal in the last decade has shed new light on the effects of DC stimulation on the human brain. A number of neurophysiological studies have demonstrated the efficacy of tDCS for the treatment of patients suffering from neuropsychiatric diseases, such as stroke, chronic pain, and depression.10 Moreover, recent studies have reported that the use of tDCS on specific brain regions can improve cognitive functions, such as attention span, working and long-term memory, language, and mathematical ability of healthy subjects.11
For example, de Vries and colleagues showed that anodal tDCS over Broca's area—a region in the frontal lobe of the hemisphere of the brain—improves artificial grammar learning.12 Other researchers have shown that ‘numerical learning was enhanced by anodal tDCS of the parietal cortex, and this effect was stable for at least 6 months after training’.13 Also, subjects who received anodal tDCS on the right anterior temporal lobe while naming pictures of famous people showed improved retrieval of proper names.14 In one study funded by the US Defense Advanced Research Projects Agency, subjects who received anodal tDCS on right inferior frontal and right parietal cortex showed learning and performance improvement in a video game designed to train military personnel to accurately identify obscured and concealed objects in a complex environment.15
These interesting experiment results began to draw the media's attention—a New York Times article in October 2013 described tDCS devices as ‘jump starter kits for the mind’.16 Also, the number of tDCS articles published per year has been growing rapidly,17 and currently at least 224 clinical studies on tDCS are going on around the world.18
On top of the fact that it can be an effective tool for both treatment and cognitive enhancement, tDCS has two other especially appealing features—it is relatively safe and affordable. Most of the known side effects of tDCS are minor adverse effects, such as headache, or slight tingling or itching under the electrodes.19 At least nine tDCS devices for personal use can be purchased through websites as a complete unit with prices between approximately  200 and
200 and  400.20 But for less than
400.20 But for less than  40 for parts and basic schematics from the web, people can build their own tDCS devices.
40 for parts and basic schematics from the web, people can build their own tDCS devices.
Thanks to these special features of tDCS, in recent years, a group of people who built or purchased the tDCS device for home use has appeared. Researchers have expressed concerns about this so-called do-it-yourself (DIY) use of tDCS. They have argued that even if there are no serious side effects, potential risks of misusing this device should not be ignored.21
For example, lay people who are DIY users may not have sufficient knowledge on the structure of brain to place the electrodes accurately, causing unintended effects.22 Moreover, reversing anodal and cathodal electrodes placement could lead to impairment of the brain by switching areas affected by excitatory and inhibitory stimulation.23 It is also possible that tDCS interacts with other treatment that DIY users are undergoing, such as psychoactive medication, in detrimental ways.24 Lastly, using tDCS to enhance some of the cognitive functions may adversely affect other functions of the brain, and potential long-term (side) effects of tDCS on the brain have not been fully explored.25
The possible need for a regulatory regime for tDCS
The FDA's own description of its regulatory regime for medical devices reads:
If a product is labeled, promoted or used in a manner that meets the following definition in section 201(h) of the Federal Food, Drug, and Cosmetic (FD&C) Act, it will be regulated by the Food and Drug Administration (FDA) as a medical device and is subject to premarketing and postmarketing regulatory controls.26
(h) A device is:
an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
(1) recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
(2) intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
(3) intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its primary intended purposes.27
‘FDA requires all medical products manufacturers to register their facilities, list their devices with FDA, and follow general control requirements’.28 Medical devices are classified in three categories—low (Class I), moderate (Class II), and high-risk (Class III) devices—according to the risks that the devices present.29 Devices with low risk are considered as exempt from FDA's pre-market review and can be legally marketed upon registration.30 In terms of moderate- and high-risk devices, there are two administrative paths that manufacturers must take to bring the devices to markets.
The first path is pre-market approval (PMA), which ‘consists of conducting clinical studies, submitting pre-market approval (PMA) application and requires evidence providing reasonable assurance that the device is safe and effective’.31 Usually novel and high-risk devices are subject to this process, and it is known to be very lengthy and expensive.32 The other path involves ‘submitting a 510(k) notification demonstrating that the devices is substantially equivalent to a device already on the market (a predicate device)’ and results in FDA clearance.33 This path, which tends to be less expensive and time-consuming than PMA, is available for moderate-risk medical devices not exempt from pre-market review.34 ‘Once approved or cleared for marketing, manufacturers must comply with regulation on manufacturing, labeling, surveillance, device tracking, and adverse event reporting’.35
Some neurostimulation devices are already classified and regulated as medical devices by FDA. For example, in 2011, repetitive transcranial magnetic stimulation (rTMS), another non-invasive neuromodulation that generates electric currents using electromagnetic coils, was classified as a Class II medical device for treatment of major depressive disorder.36 Also, cranial electrotherapy stimulation, whose mechanism is similar to tDCS except that it applies alternating current, not DC, to the brain, has been regulated as a medical device (Class III) for treatment of insomnia, depression, or anxiety since 1979.37
Inconsistently, however, tDCS is not covered by the current regulatory regime. FDA guidance explicitly provides that ‘there is no regulation for the therapeutic tDCS’.38 A few clinics offer tDCS treatment to their patients with various medical conditions, such as depression and chronic pain, but these clinics apply tDCS as ‘off-label’ use of the iontophoresis device—a DC stimulator for introducing ions of soluble salts or other drugs into the body for medical purposes that FDA classified as a medical device (Class II/III) in 2004.39 In addition, devices marketed for cognitive enhancement are not covered by any existing legislation in the USA.40
In their recent article, Nick Fitz and Peter Reiner stated that this regulatory vacuum is understandable ‘given that the myriad applications of tDCS are fairly new’.41 However, since tDCS devices are already on the market and being sold to the public, they argued that now we should begin the discussion on how to develop a regulatory policy for the DIY use of tDCS. For example, foc.us, a fancy tDCS device marketed as a gamer headset to boost attention, completely sold out its first batch of 3000 units less than a month after its release in May 2013.42
Fitz and Reiner urged ‘all stakeholders—regulators, scientists and the DIY community—to share in crafting policy proposals that ensure public safety while supporting DIY innovation’.43 They emphasized the importance of communication between policy makers and DIY users ‘to develop the ethos of responsible use’ based on the idea that ‘people should have access to a diversity of opportunities created by enhancement technologies’.44
Responding to Fitz and Reiner's call for a policy debate, Maslen and colleagues proposed a regime that regulates cognitive enhancement devices such as tDCS by extending the existing legislation for medical devices.45 In line with the ‘managed technological optimism’ that Fitz and Reiner advocate, they suggested the incorporation of a ‘low-risk exemption’ for any cognitive enhancement devices falling below a given level of risk.
In June 2013, TDCS Device Kit, Inc., a tDCS device manufacturer based in California, voluntarily recalled its products after an inspection by California Department of Public Health (CDPH).46 CDPH determined that these products were not manufactured in compliance with good manufacturing practices for medical devices and that the devices lacked adequate labeling for directions for use and warnings against dangerous uses. CDPH warned customers not to use tDCS devices from this manufacturer. This was a meaningful step taken by the government authorities, but it only had power in one state as there is no regulation on tDCS under the current FDA regime for medical devices.
The certain need for information about tDCS use
Despite the recent surge of attention to the potential risks and regulations of tDCS, there has been no attempt to systemically study what is really happening in the DIY tDCS user community. Discussions of issues related to the DIY use of tDCS are mostly based on speculation, which may lead to importantly incorrect understanding of the DIY community and its use of tDCS.
This study is the first exploratory research on who DIY tDCS users are and what is happening in this community. Specifically, this study aims to answer following four questions on the DIY use of tDCS through an online survey, a content analysis of web postings, and interviews with the DIY users.
What are the general demographic characteristics of DIY users?
Why and how do they use this device?
What are the perceived effects or side effects of this device?
What are their concerns, if any, about the DIY use of this device?
The study does have some substantial limitations. In particular, its survey was not of random users but of a set of self-selected DIY tDCS users who responded to an invitation to participant the author posted on the two major DIY tDCS internet web sites. Also, only a small number of people interviewed, chosen from among those who, based on the websites, appeared to be influential in the DIY tDCS user community. The unregulated, unregistered, and otherwise unreachable nature of the community of tDCS users made these the only feasible approaches, but they do undercut, to some extent, the confidence that can be placed in the findings. Nonetheless, even if viewed as only preliminary data, these results provide some basic information on DIY tDCS users and their attitudes and practices of DIY tDCS which will help us to lay the groundwork for designing sound future policy and official guidelines on tDCS.
Section 1 describes the three research methodologies recruited in this study, and the findings on the DIY tDCS user community are presented in Section 2. Section 3 explores potential legal and regulatory implications of these findings considering possible regulatory options for the DIY use of tDCS. Finally, Section 4 summarizes the paper's major findings and briefly discusses future directions for research.
RESEARCH METHODOLOGIES
There has been no official report on the size of the DIY tDCS user population in the USA, in any other country, or worldwide. Users do not need a prescription, do not need to register their use, and do not even need to purchase tDCS units. However, two major DIY tDCS Internet websites, www.reddit.com/r/tDCS and www.diytdcs.com, where DIY users usually visit and share information about tDCS are potential resources for exploring DIY tDCS users. The site, www.reddit.com/r/tDCS/ (hereinafter subreddit tDCS), is an open forum for tDCS users and www.diytdcs.com (hereinafter diytdcs) is a personal blog by a famous lay expert on DIY tDCS. The research subjects of this study are the registered users or visitors of these two websites (1) who built or purchased their personal tDCS devices and (2) who have used them for treatment or as a cognitive enhancer.
The research methodology of this study has three parts: (1) an online questionnaire survey, (2) a content analysis of web postings on the DIY use of tDCS, and (3) in-depth interviews.47
Online questionnaire survey
The first part of the overarching research strategy is an online questionnaire survey. The survey was composed of a minimum of 21 to a maximum of 29 questions—depending on the respondents’ answers to some questions—on basic demographic information of DIY users, their aims of practicing DIY tDCS, current practices of tDCS (including types of devices, stimulation protocols, and frequency of use), effects and side effects of tDCS, and their concerns about the DIY tDCS (See Appendix A for the questionnaire). The survey was posted on subreddit tDCS from December 27, 2013 to March 10, 2014 and on diytdcs from December 29, 2013 to March 10, 2014. Valid complete responses were collected from 121 people.48
The responses of this online survey do not form a random sample, and there is a potential bias because these respondents are self-selected to participate in this survey. However, since this study is the first preliminary attempt to learn about the DIY tDCS user community, responses from this convenience sample should provide previously unknown but useful facts about this community.
Content analysis of web postings
The second research methodology is a content analysis of web postings on subreddit tDCS. In this well-known and very active online forum, DIY users ask and answer questions on the DIY use of tDCS device, post interesting recent tDCS studies, and share their experiences of positive and adverse effects of tDCS devices. These web postings are an important source of information to answer some of the research questions.
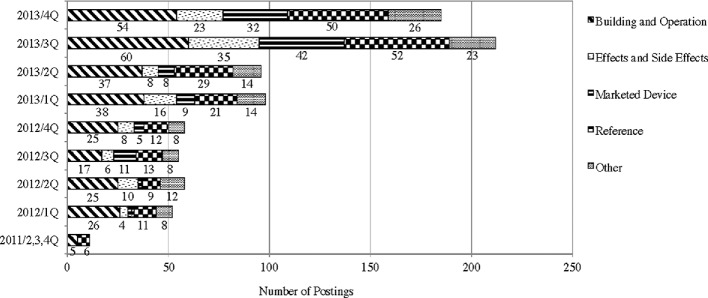
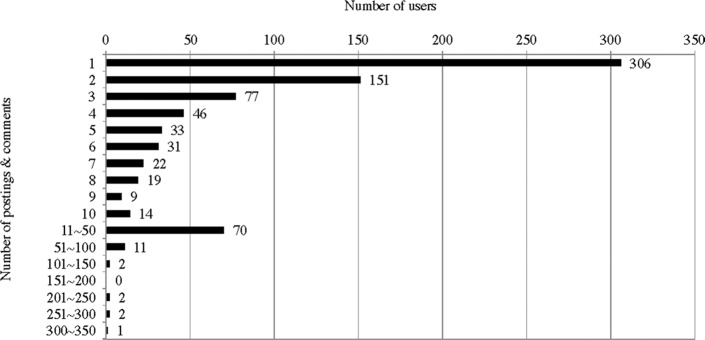
Subreddit tDCS was established in April 2011; since then, there has been a significant increase in the number of postings (Fig. 1). By the end of 2013, 796 users had made 825 postings and 4756 comments to the postings. This study took a census of all 825 postings, and the comments to these postings were selectively included in the analysis in cases where the comments showed meaningful discussions on the issues raised in the postings.
Figure 1.
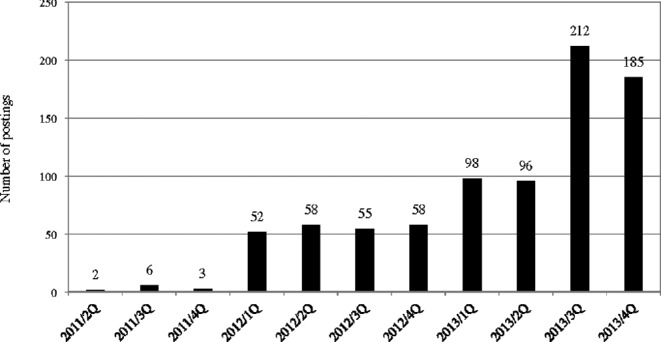
Number of posting per quarter in www.reddit.com/r/t/DCS (2011–2013).
Since there is no previously established theory or research study on the DIY tDCS user community or subreddit tDCS, the codes were derived from an emergent process.49 Through a preliminary examination of the postings, five major codes to categorize the main theme of each posting were developed: (1) building and operation of the tDCS device, (2) marketed devices, (3) effects/side effects of the device, (4) references (external links) to research papers, newspaper/magazine articles, or blog posts on tDCS, and (5) others. When there was more than one theme in a posting, this study coded the posting according to its primary purpose. For example, if a user began his or her posting with the side effect he or she has experienced, but then later asked about how to improve his or her DIY circuit to prevent this side effect, this posting would be classified under the code, ‘building and operation of the tDCS device’.
In addition to these major codes, two additional emergent codes—the commenters’ reasons for using the tDCS device and their warnings about safety– were prepared to describe recurrent topics that answer some of the research questions of this study but do not constitute the main theme of the postings. For example, DIY tDCS users usually mentioned their purpose for using the tDCS device while asking questions about building and operation of the device (See Appendix B for detailed classification of the codes).
In-depth interviews
After survey data analysis and content analysis of web postings were completed, five semistructured interviews were conducted to collect more detailed information on the user experience and users’ opinions on the official guidelines and potential government regulation of the tDCS device.
First, this study conducted a brief analysis on the user IDs and postings at subreddit tDCS to identify ‘power users’ who could provide a general overview and informative facts on the DIY tDCS user community. The analysis revealed five users who have made more than 200 postings and comments at subreddit tDCS (Appendix C). Four of these five ‘power users’ participated in the interviews: the moderator of subreddit tDCS, the Webmaster of diytdcs, a user who has uploaded popular self-experimentation videos on his YouTube channel, and a user who has been using tDCS for treatment of bipolar type II and other medical conditions.50 51 The protocol for these four interviews was prepared based on the survey questionnaire. The interviewees were also asked to give their thoughts on the major findings of the survey and content analysis as an active user or an observer who is familiar with the trend in the community. Second, a physician who has been treating about 350 patients with tDCS for the last seven years was also recruited for the interview to provide a medical perspective on the DIY use of tDCS.52
The interview with Webmaster of diytdcs was conducted through email, and the moderator of subreddit tDCS and the physician was interviewed over Skype. The other two power users were interviewed on the phone. The phone and Skype interviews were conducted for about 30 minutes to an hour and audiotaped with the consent of the interviewees.
Although the number of samples is very small, given the exploratory nature of this study, these interviews should provide a meaningful preliminary sketch of the DIY tDCS community and current uses of tDCS along with the findings of the survey and content analysis of the web postings.
FINDINGS
In this section, the results of the survey, content analysis of web postings, and in-depth interviews will be presented and analysed under the four main research topics of this study: general demographic characteristics of DIY users; their reasons for and current uses of tDCS; effects and side effects of tDCS; and their concerns about the DIY use of tDCS.
Demographic characteristics of the DIY tDCS user community
There is no official estimate of the size of the DIY tDCS user population. As of the end of 2013, subreddit tDCS had about 2700 subscribers. According to the moderator of the website, there were 4000 individual hits on this forum per month in 2012. The number has been gradually increasing, and this forum is now getting 6000 individual hits per month. Although it is very hard to track whether a particular person is really using tDCS, the moderator estimated that ‘6000 might be the upper bound of the DIY tDCS user population and the lower bound might be the number of subscribers to this website, about 3000’, assuming that an active, regular tDCS user will visit the website at least once a month.
One of the power users who has posted self-experiment videos said that 800 people were subscribing his YouTube channel, and another famous YouTube channel on tDCS has 2700 subscribers. This power user estimated that the size of the DIY tDCS user population is probably a few thousand, but not more than 10,000.
However, the Webmaster of diytdcs, who is also one of the most active participants in subreddit tDCS since the establishment of that forum, questioned this estimate of the size of the DIY tDCS community in the media and academic studies:
I'm not sure how large the community is just now. It seems people pop in for a period and then move on, though there are also committed DIY tDCS users who are self-treating daily. I'm really just not sure of the numbers. … There has been a lot of hype around DIY tDCS. The community may not in fact be very large. … My sense is that tDCS is a fringe interest that won't attain popularity unless some sort of yet to be seen benefit emerges. (For example, that a tDCS montage could facilitate retrieving early memories).53
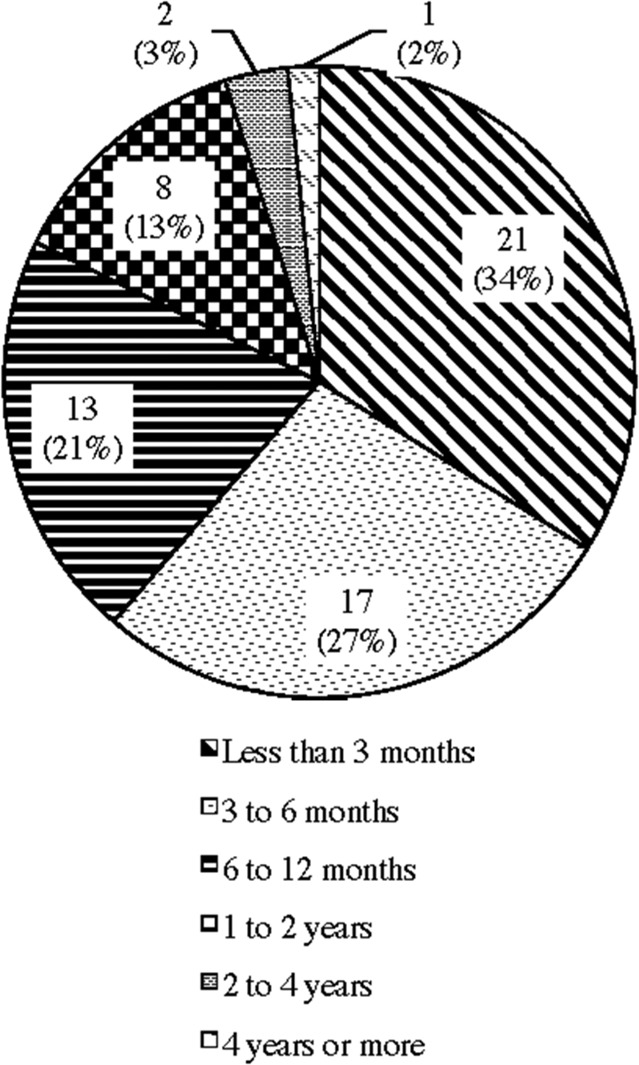
The survey results seem to support the Webmaster's view. Only half (62; 51%) of the respondents reported that they are continuous and regular tDCS users and among these continuous and regular users, 61% of them have been using tDCS less than six months (Appendix D). The majority of the respondents who claimed that they are continuous and regular users began to use tDCS pretty recently.
Thus, although, based on evidence of internet uses, the DIY tDCS user population seems to have been growing for the last few years, these rough indicators might suggest that the dedicated tDCS user population has been overstated. ‘There seems to be a small core of dedicated DIY tDCS users that enable a sustained presence at the tDCS subreddit. But apart from that a lot of people come in for a quick look and then wander off’.54
Whatever the community's actual size, the results on the demographic characteristics of the surveyed community are analysed below.
First, the survey respondents are overwhelmingly male—114 respondents (94%) are male, 5 respondents (4%) are female.55
Second, most of the respondents are in their 20s and 30s (86; 71%), but there are a significant number of respondents in their 50s and over (15; 12%) (Fig. 2). The physician who has been treating patients with tDCS reported that there are some elderly patients who want to restore their diminishing cognitive abilities. He argued that tDCS is an appealing brain stimulation technology not only for young self-experimenters or bio hackers but also for seniors suffering from deterioration of mental functioning. Also, it is noteworthy that there are some minors using tDCS (7; 6%).
Figure 2.
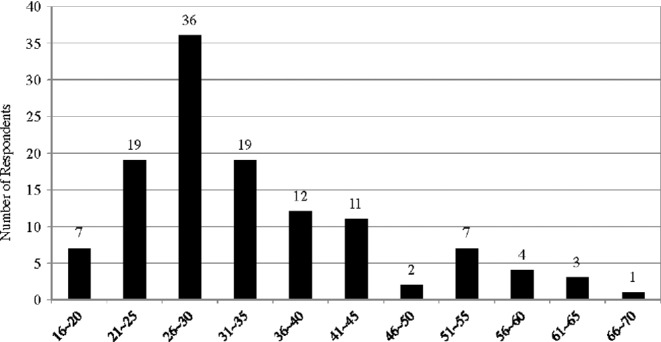
Age of respondents (year).
In terms of country of residence, most of the respondents (90; 74%) are from North America—the USA and Canada, although the rest of them are widely spread around the world.56
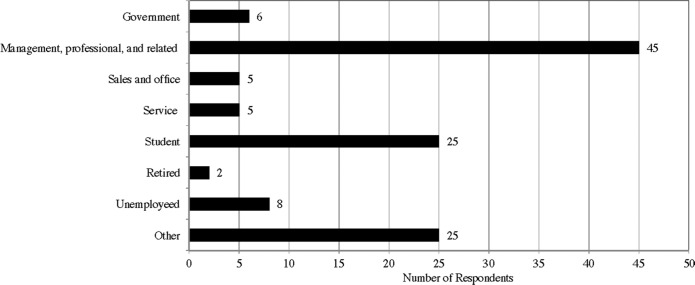
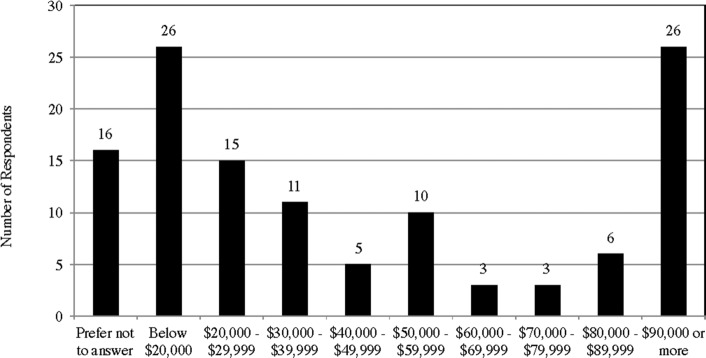
In addition, the majority of the respondents have four-year undergraduate degree or Masters degree (62; 51%), though a significant number of respondents only have a high-school degree or below (29; 24%) (Appendix E). Relatedly, most of the respondents are students (25; 21%) or professionals (45; 37%) (Appendix F). The reported annual income level of the respondents also seems to fall in line with their occupations—the respondents either have reported income of less than  20,000(26; 21%) or reported income of more than
20,000(26; 21%) or reported income of more than  90,000 (26; 21%) (Appendix G). However, making generalizations on respondents’ socio-economic status based on these data can be problematic given that the respondents are from all over the world.
90,000 (26; 21%) (Appendix G). However, making generalizations on respondents’ socio-economic status based on these data can be problematic given that the respondents are from all over the world.
Reasons for and current uses of tDCS
This part will delineate and analyse the data on DIY users’ aims of using tDCS device, as well as their current use of tDCS—such as types of tDCS devices, stimulation protocols, and frequency of use.
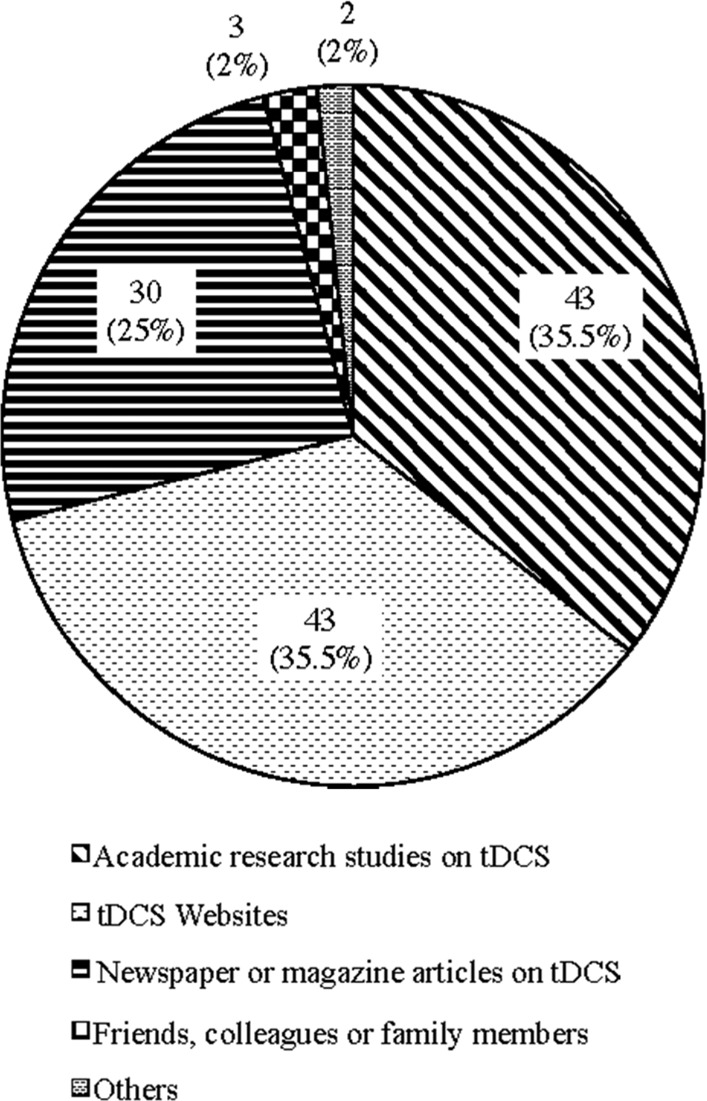
First encounter with tDCS
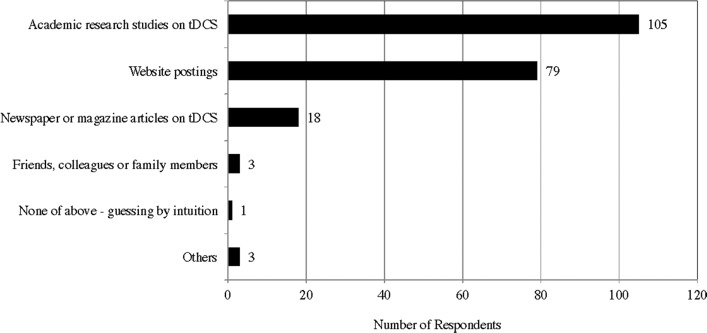
According to the survey results, respondents first learned about tDCS through academic research studies on tDCS (43; 35.5%), tDCS websites (43; 35.5%), and newspaper or magazine articles on tDCS (30; 25%) (Appendix H). This indicates that information on tDCS is mainly disseminated and shared through online media and forums, assuming that many of respondents may not have direct access to academic journals, and recent intriguing studies on the various effects of tDCS have been vigorously covered by the media.
The result of content analysis also concurred with this finding. Fig. 3 presents the number and the percentage of subreddit tDCS postings classified under the five codes. This shows that this website functions as a forum for discussing how to build and use tDCS and sharing new scientific discoveries on the effects of tDCS featured in media and various online forums.
Figure 3.
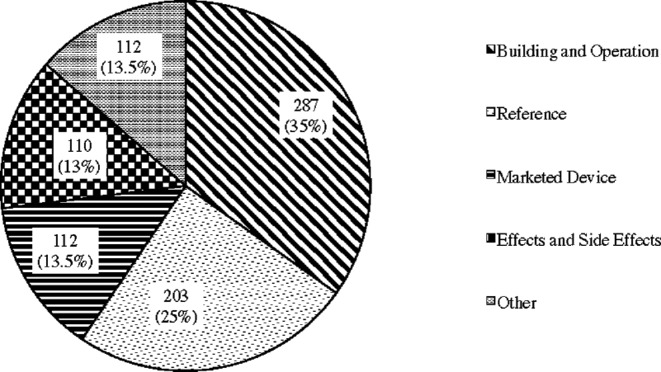
Postings at subreddit tDCS.
Reasons for practicing tDCS
A total of 13 (11%) respondents use tDCS for treatment of medical conditions and 71 (59%) respondents use it for cognitive enhancement. A total of 29 (24%) respondents reported that they are using tDCS for both cognitive enhancement and treatment.57,58 The most common types of cognitive enhancement purpose for which the respondents use tDCS were attention (72), learning (motor, verbal, or numerical learning, etc.) (68), and working memory (67) (Fig. 4).59
Figure 4.
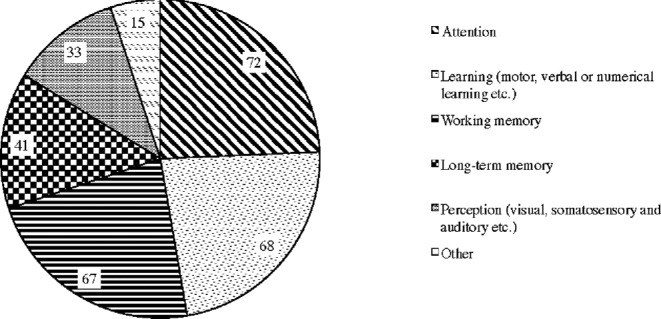
Types of cognitive enhancement purpose for which respondents use tDCS.
In addition, the most common medical condition for which the respondents use tDCS was depression (Fig. 5).60 Interviewees of this study also reported that tDCS is very effective for the treatment of depression, especially for treatment-resistant depression. According to the physician who has been treating patients with tDCS, most of his patients came to the clinic because they do not respond to psychoactive medications. Based on his extensive clinical experiences, he claimed that tDCS is significantly beneficial for the treatment-refractory patient suffering from chronic depression—‘it takes only about five treatments before the effects of tDCS become noticeable’. One of the power users who has bipolar type II disorder also reported that ‘the effect of tDCS is immediate’ and he ‘can even tell when the machine is shutting off’.
Figure 5.
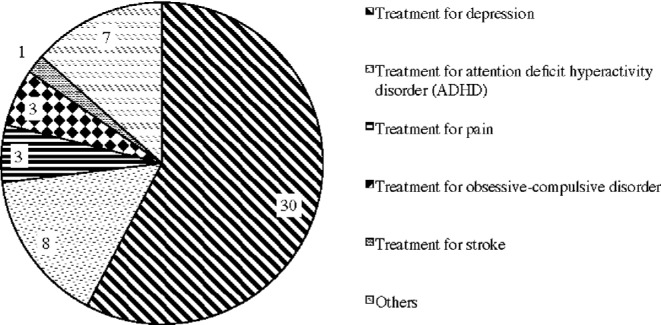
Types of medical condition for which respondents use tDCS.
Furthermore, this survey also asked, in cases where the respondents are using tDCS for treatment, whether these respondents are also taking other conventional medications. This question is intended to assess the risk of DIY tDCS, assuming that there could be unknown adverse compounding effects of tDCS and other extant medications. ‘The pharmacological status of the brain can have meaningful effect on the outcome of tDCS, and the variety of psychoactive agents that home users may employ is legion’.61
In this survey, 18 respondents—44% of respondents who are using tDCS for treatment of medical conditions—reported that they are currently taking other medications for the same medical conditions.62 Thus, a significant percentage of respondents who are using tDCS for treatment are exposed to the potential detrimental interactions with other medications.63
Types of tDCS device
The survey result showed that among the 121 respondents, 47 (39%) respondents built their own device and 58 (48%) respondents bought ‘out of the box’ tDCS devices. Some of the respondents (11; 9%) reported that they both built and bought their devices.64
In terms of home-built tDCS devices, the analysis of subreddit tDCS postings revealed that some open source tDCS circuit designs proposed by DIY users had been available at this forum and improved through active discussions among users. OpenStim tDCS, by the moderator of subreddit tDCS,65 and another designed by a user named Shawn Nock, are the two most well-known open source tDCS circuits.66 The moderator of subreddit tDCS initially had wanted to buy one of the devices used at clinics or research labs but the prices of these devices were over his budget.67 After he found out that building a tDCS device does not require complicated knowledge of electrical engineering, he decided to design his own circuit, which is affordable (costs less than 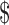 50) and, he believes, safe, and to share it with other DIY users.68 Later, ‘it has been evolved with the inputs from other users in the community in terms of what kind of features would be useful’.69
50) and, he believes, safe, and to share it with other DIY users.68 Later, ‘it has been evolved with the inputs from other users in the community in terms of what kind of features would be useful’.69
In addition to these open source circuits, some users also compiled and posted basic schematics, tools and electrical components needed to build the tDCS device, and online or offline shops that one could get those tools and components, as well as their prices.70 Thus, at subreddit tDCS, instructions on how to build an easy-to-use tDCS device at a very low cost are easily accessible to anyone who is interested in trying self-experimentation with tDCS.
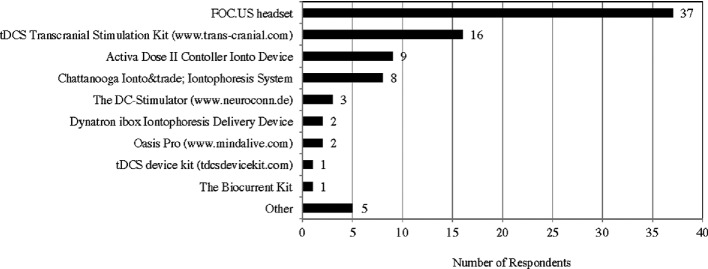
Regarding the ‘out of the box’ tDCS devices, currently about 10 devices are on the market. The survey result showed that the most prevalent device in this community is the foc.us headset. (Fig. 6)71,72
Figure 6.
Types of ‘Out of the Box’ tDCS device that respondents are using.
However, both power users and the physician had unfavorable opinions on the foc.us headset, mostly because its placement of electrodes, which is called montage, is fixed.73 The physician argued that the montage of foc.us device is different from montages used in any of the clinical studies, and he did not think it has the same effects as the ordinary two electrodes system using iontophoresis devices that researchers have used in finding evidence of effectiveness. The Webmaster of diytdcs also stated that ‘unless some sort of tangible evidence of obvious benefit emerges, foc.us will turn out to be a fad and will most likely fade away’.
The fact that these commercial devices, such as foc.us, are gaining popularity among the DIY users has caused a demographic shift in this DIY tDCS community. The moderator of subreddit tDCS recalled that two years ago—when the website was first established—people were talking about ‘designing electronics, designing techniques, and designing experiments’. However, ‘there is now a population of people who are basically end users and are not programmers, hackers or electronics people’.74 This is also reflected in the recent sharp increase in the number of postings on these commercial devices at subreddit tDCS (Fig. 7). The moderator argued that ‘this has good and bad sides’. It is good in the sense that many of these ‘out of the box’ products are ‘quite sophisticated’. For example, the foc.us device can deliver custom waveforms, which is difficult with DIY devices. If commercial devices become more popular, they can spur people's interest in developing tDCS methods that can take advantage of the more advanced functionality. On the other hand, the moderator said we cannot assume that these end users are knowledgeable enough to make informed decisions about their safety in regard to issues like whether a specific type of circuit is safe to use in these devices, and thus, ‘it puts more responsibility on the manufacturers’.75
Figure 7.
Trends in the postings at subreddit tDCS.
Elements of stimulation protocol: intensity of current, size of electrodes, and duration of stimulation
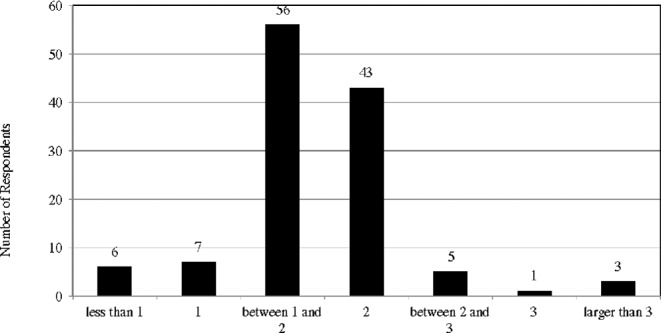
Three factors determine the safety of a tDCS protocol: dose of current, size of electrodes, and duration of stimulation.76 The first two factors determine current density (current dose divided by electrode size), and the total current dosage is then measured by multiplying current density by the duration of stimulation.77 Researchers have suggested that a stimulation protocol using 25–35 cm2 electrodes with currents of 1–2 milliamperes (mA) for up to 20–40 minutes is considered safe.78 The survey results showed that most of the respondents are following this safety guideline, though there are some exceptions. (Figs 8–10) For example, 43 respondents are using electrodes smaller than 10 cm2. However, it seems that most of them are foc.us users: the size of electrodes of foc.us headset is about 4–6 cm2, and there are 37 foc.us users among the respondents.
Figure 8.
Dose of current (mA).
Figure 10.
Duration of simulation (minutes).
Figure 9.
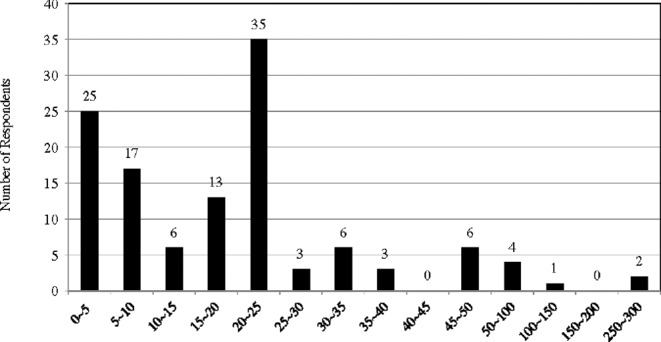
Size of electrodes (cm2) .
When asked how they try to find out about these three factors that determine the safety of a stimulation protocol, most respondents answered that they find relevant information from academic research studies on tDCS or website postings, such as postings in subreddit tDCS (Appendix I).79
Frequency of use
How often one can safely use tDCS per day or per week for an extended period of time is a question that does not have a clear answer yet. Most of the tDCS studies applied tDCS to the subjects less than five to six times per week for a relatively short term (ie few weeks).80 Thus, these studies do not provide proper guidelines for people who are using tDCS at home more often for a longer period of time. A few postings on the frequency of use at subreddit tDCS led to users replying based on their own experiences and acknowledging the absence of proven guidelines by researchers.81 A total of 52 out of 56 respondents who are using tDCS regularly reported that they have stimulation sessions fewer than seven times per week (on average less than once a day).82
The self-perceived effects and side effects of tDCS
This part presents, the data on the respondents’ self-reports about the effects and side effects of tDCS. In this exploratory study, it is not feasible to measure and test the actual effects of tDCS, such as to what extent tDCS has affected users’ cognitive abilities or medical conditions.
The self-reports on the effects of tDCS posted at subreddit tDCS show that DIY users themselves are having problems in assessing the effects of tDCS while using it at home.83 Although the physician who has been treating his patients with tDCS described the effects of tDCS on chronic pain and depression as ‘too good to be true’, a power user who used tDCS mainly for cognitive enhancement reported that the effects of tDCS are ‘extremely subtle’. For example, there can be placebo effects and in cases where users take cognitive tests after or while simulating their brain (ie Lumosity, Dual N-Back Game, or Cambridge Brain Challenge), 84 learning by repetition can also affect the test results.
Thus, this study will focus on users’ subjectively perceived effects of tDCS. In addition, types of side effects which DIY users are experiencing and the degree of these side effects will be analysed.
Effects of tDCS
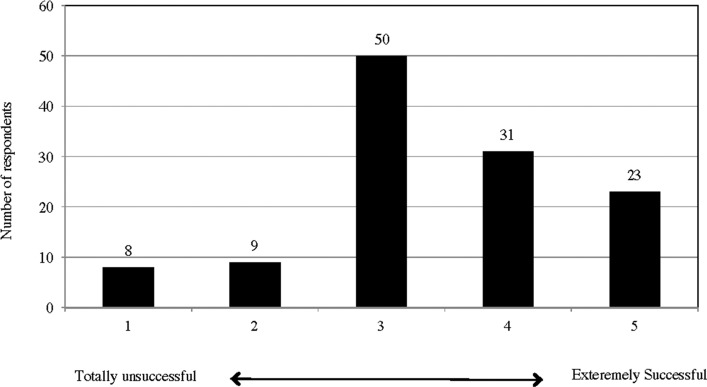
A total of 54 (44%) out of 121 responding users rated the effects of tDCS at four or five on the scale of one (totally unsuccessful) to five (extremely successful) (Appendix J).
The quotes below are two examples of self-reports on the effects of tDCS posted at subreddit tDCS. The first quote is describing the effects of tDCS on cognitive abilities:
A series of memory tests were administered by an electrical engineer who supervised the experiment, initially without tDCS. These tests demonstrated standard working memory results with a measured span of 5–8 components retained after a series of 15 terms were listed aloud to remember. … With tDCS the data showed very promising results. Apart from the subjective experience of heightened focus and a calmness that can be likened to being in the zone, the results of the memory test indicated a measurable increase in working memory span. For each subsequent test, all 15 words were remembered in correct order and recalled in correct order after tDCS was administered. … [T]he subjective experience combined with data reflecting very promising cognitive enhancements leads me to conclude tDCS overall effectiveness in increasing my learning potential during and post stimulation.85
The second quote describes the perceived effects of tDCS on a medical condition—depression:
From a subjective perspective, this is awesome. Best night of my life awesome. I actually went out jogging. Mostly walking, actually, because there isn't enough electricity in the world to make me not be a fat ass who's running for the first time since 2010, but still way out of my usual. I initially questioned if this was a hypomanic episode or just what the release of 15 years of depression feels like, but I judge it to likely be hypomania. Doesn't matter, I feel like Frankenstein's monster. I was dead, if only in my mind, and then with a little bit of lightning, boom. I'm alive.86
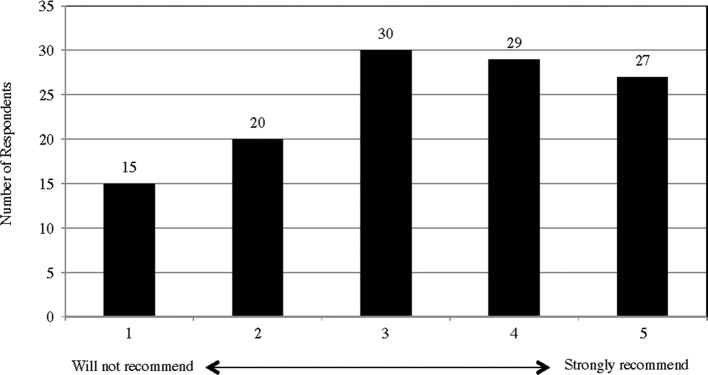
As an additional indirect measure of subjectively perceived effects of tDCS, survey respondents were asked whether they are planning to use tDCS in the future. A total of 111 out of 121 respondents (92%) replied that they would continue using tDCS in the future. Moreover, 56 (46%) of the respondents rated their willingness to recommend tDCS to family members or friends, who have the same aim(s) or conditions(s) as they do, at four or five on the scale of one (will not recommend) to five (strongly recommend) (Appendix K).
Side effects of tDCS
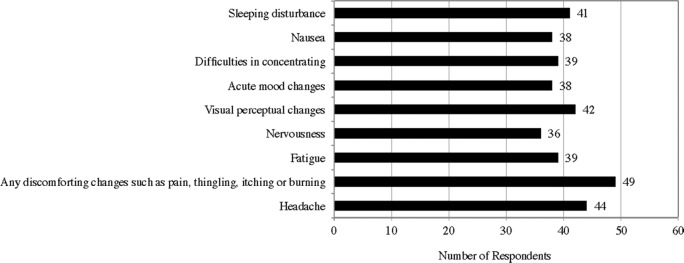
A total of 56 out of 121 responding users (46%) reported that they have experienced side effects while using tDCS. These respondents have experienced headache, discomforting changes such as pain, tingling, itching or burning under the electrodes, fatigue, nervousness, visual perceptual changes, acute mood changes, difficulties in concentrating, nausea, and sleeping disturbance (Appendix L).87
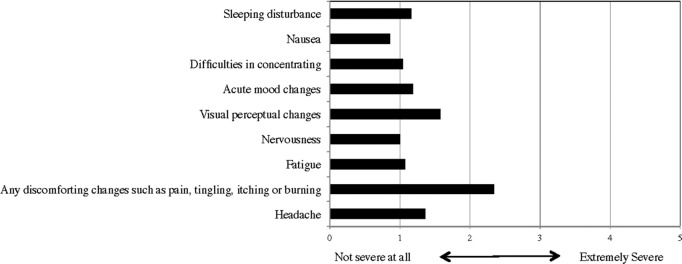
However, the degree of side effects was not severe—the average severity of most types of side effects were rated at about one, on the scale of one (not severe at all) to five (extremely severe), except discomforting changes, such as pain, tingling, itching or burning under the electrodes, where the average severity was about 2.3 (Appendix M). Power users and the physician reported that the side effects of tDCS are almost negligible, and this result corresponds to previous empirical results showing that there are no severe short-term adverse or side effects.88,89
Concerns about the DIY use of tDCS
What concerns did DIY users have, if any, about the use of tDCS are and what they think about the possibility of government regulation on tDCS or guidelines from government agencies, researchers, or physicians?
Concerns about the DIY use of tDCS
A total of 97 out of 121 survey respondents (80%) reported that they have concerns about the DIY use of tDCS. Most of these respondents are worried about long-term potential impairment on the brain and the use of inaccurate position of electrodes (Fig. 11).90
Figure 11.
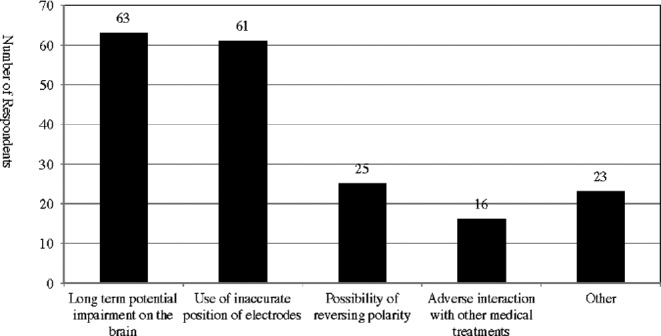
Concerns about the DIY use of tDCS.
The most interesting finding in the analysis of web postings at subreddit tDCS was the users provided safety warnings. In subreddit tDCS, 100 safety warnings were found in the comments to the postings, and 77 out of these 100 warnings were the comments to the postings on building and operation of tDCS (Table 1).
Table 1.
Safety warnings in the comments to the postings.
| Theme of the posting | Number of safety warnings in the comments to the postings |
|---|---|
| Building and operation | 79 |
| Effects and side effects | 6 |
| Marketed device | 7 |
| Reference | 4 |
| Other | 4 |
| Total | 100 |
Below are the examples of safety warnings.
‘If you have “no idea what a 4 milliamp is”, I suggest you stay away from this. A 9V battery CAN kill you!’
‘Remember safety first before you try this’.
‘Don't up the current, that is the wrong way around’.
‘Seriously, if you aren't comfortable with your knowledge of currents and what effects different amperages can have on your body, do not attempt this’.
Sometimes DIY users also ask more specific questions on their own DIY circuit at subreddit tDCS.91 They post pictures or detailed descriptions of their home-built circuit and ask whether it is safe to do self-experiment with the circuit. Then, other users actively correct them if there are issues on the circuit that can cause danger.
The general safety warnings and questions and comments about the safety of a specific DIY circuit design show the existence of a self-regulating system within this online forum. The Moderator of subedit tDCS stated that ‘there is a lot of emphasis on individual responsibility—a bio hacker's mentality, but there is also a very much of a culture that values giving people information about dangerous things’. The Webmaster of diytdcs also acknowledged that there is ‘definitely’ a self-regulating system in subreddit tDCS: ‘Those who do know about electronics easily recognize people who shouldn't be experimenting with self-built devices’.
Other power users also agreed that there were a certain number of users in the community who have corrected and guided other users not to make dangerous mistakes:
We are self-regulating community. … Everybody in Reddit has to express some type of knowledge as he/she writes a posting and nobody in the community will let up on you understanding the dangers of messing around with electronic current. The reason that it happens is because I believe at least in the beginning most of the tDCS community on Reddit were people who had a background in engineering such as building circuits. These people were extremely helpful in giving real advices. Over the course of last three years, I have yet seen anybody post anything that tDCS has done some real damage. So, I guess it (the self-regulating system) is working.92
DIY users’ thoughts on potential official guidelines and government regulations
The majority of survey respondents (97 out of 121 respondents) thought that it would be helpful to have an official guideline from government agency or experts (researchers or physicians) for the DIY use of tDCS. All of the power user interviewees claimed that the part that most needs guidelines from the government or experts is placement of electrodes. They reported that getting reliable repeated placement of anodes and cathodes, and what montages should be used to treat or improve which things are the most confusing parts of practicing DIY tDCS. Moreover, the power users expressed concern that even though DIY users locate the electrodes in the right place according to a specific montage, whether a person is left-handed or right-handed might affect the outcome because left-handed people's brains may be organized differently from those of right-handed people.93 ‘It is also possible that subtle differences in the neuroanatomy of individuals may alter the effects of the device’.94 The physician also warned that the area under the anode that is being stimulated could be changed depending on where you locate the cathodes.
Some of the survey respondents also provided more detailed comments on the potential official guidelines or government regulation on tDCS.
‘Official guidelines would only be helpful if they were more than “don't do it”. I think in many cases our society focuses too much on safety rather than encouraging curiosity’.
‘I believe that government regulation would be a detriment and stifle any new developments’.
‘tDCS has great potential. I'd like guidelines to come out that are backed by proper and thorough research into the effects’.
‘While I think more information and studies being published from academic and medical sources would be great, I hope that the industry does NOT undergo any governmental regulation, and remains a practice individuals can perform on themselves and buy the related devices without legal issues’.
‘The use of these technologies will evolve and outpace the medically established research merely based on the network effect that the Internet creates around anything it touches. We need to create a sort of social network for experimenters to share and teach each other. Gov't guidelines will be suspect and overly safe and completely unheeded. If they are smart, they will study and not interfere except to add useful insight from time to time from their own research’.
‘About official guidelines–I'd welcome any helpful information, but such guidelines, in my experience tend to be over-cautious. I can't imagine any official agency ever advising the public to do what I do. I wouldn't advise it, myself. My own experience has been very satisfactory, but I know that there are a lot of unknowns and that I'm accepting an unknown measure of risk when I run an electrical current through my cranium. Nobody should do that until they've researched the matter thoroughly and assessed the risks for themselves. What I would like is an advisory agency for psychologists and psychiatrists to help them deliver tDCS treatment where it's appropriate. I think it can be very beneficial, and not many professionals seem to be aware of it’.
‘I have great concerns with some of the equipment people are using to attempt this on their own brains. I'm an electrical engineer so when I look at some of the devices people have designed it's terrifying what these people are hooking up to themselves. It would be very, very useful for an official guideline to be available since some of these people are using simple voltage dividers with no understanding how current behaves when they apply these things to their heads’.
These comments suggest that although DIY users acknowledge the need for guidelines for the DIY tDCS; many are opposed to and worried about blanket government regulations on tDCS devices, which would prevent them from continuing self-experiments. These blanket regulations could ‘backfire and draw further attention to DIY tDCS’,95 and lead to the opening of uncontrollable underground markets for tDCS.
According to the interviewees, one of the most serious concerns about government regulations seems to be the potential increase in the cost of practicing tDCS. The moderator of subreddit tDCS said that the community has a strong preference for devices that are extremely low cost. ‘It is very difficult to convince people to have a current meter on the home-built device because it costs 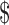 5 more’.96
5 more’.96
Criticizing the current US health care system that discourages physicians from reducing the costs of medical treatments, the physician also argued that other available options, comparable to tDCS, for treatment refractory patients are too expensive. For example, it costs 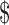 20,000–
20,000– 30,000 per year for the treatment of chronic depression using rTMS, which is a classified medical device by FDA, and insurance often does not cover the treatment. In contrast, his clinic offers a tDCS treatment program at
30,000 per year for the treatment of chronic depression using rTMS, which is a classified medical device by FDA, and insurance often does not cover the treatment. In contrast, his clinic offers a tDCS treatment program at  2500, which includes four years of at-home supervision, as well as the initial evaluation, training, and a device—although it still could be considered expensive for some patients. In other words, the costs could be the main reason why people with specific treatment refractory medical conditions, such as chronic pain or depression, opt to build or purchase a tDCS device and self-treat them. These people might view a strict regulatory oversight as a threat to deprive them of the only affordable last resort to keep their medical condition under control.
2500, which includes four years of at-home supervision, as well as the initial evaluation, training, and a device—although it still could be considered expensive for some patients. In other words, the costs could be the main reason why people with specific treatment refractory medical conditions, such as chronic pain or depression, opt to build or purchase a tDCS device and self-treat them. These people might view a strict regulatory oversight as a threat to deprive them of the only affordable last resort to keep their medical condition under control.
As a last note, below quote from the interview with the moderator of subreddit tDCS suggests an interesting angle for a future regulatory regime regarding tDCS:
If the goal of regulating tDCS device is to make sure that when people are getting tDCS device, it is safe and effective, I think labeling and regulating it as a medical device is not practical and that's because they are so easy to make. The medical device testing is onerous and expensive and not something that many of the DIY users are going to like to go through … So, there are always going to be a supply of unregulated devices and you cannot stop people from making this device. It would be better to adopt a wide-spread and easily understandable standard system for tDCS that is based on research on safety of tDCS and have a group to test tDCS devices to make sure that the devices meet the standard—you would think twice about buying a car that has one star crash rating, and I would like to see something like that applies to tDCS devices as well.
TENTATIVE REGULATORY IMPLICATIONS OF THE FINDINGS
The findings of this study presented in the last section may provide preliminary empirical indicators to determine whether we need regulations and guidelines for DIY tDCS and, if we do, how to design them to ensure a safe and responsible use of tDCS. This section will summarize some of the main findings and explore the tentative regulatory implications of these findings.
Current state and future prospects of the DIY use of tDCS
First, unlike the implicit assumption made in media coverage on tDCS, the DIY community does not comprise just risk takers with eccentric interests, such as young bio hackers or self-experimenters. The survey results showed some distinctive characteristics of the respondents—clear male dominance and slight skew to (a) higher than average education level, (b) students and professionals, and (c) very low and high income. Also, it should be noted that there was a wide distribution of ages among the respondents, which shows the potential of tDCS as an appealing technology satisfying various aims across generations. Although the survey respondents did not necessarily form a representative sample, this demographic profile of DIY tDCS users provides preliminary information on who will be the target subjects of potential regulations or official guidelines on the use of tDCS.
Second, according to the findings of the study, DIY users’ interest in tDCS seems transient—only half of the survey respondents are continuous and regular users, and the majority of these respondents (61%) began to use tDCS less than six months ago. One of the interviewees also pointed out that there seem to be only a small number of committed DIY users in the community. From this data, it can be inferred that there may be some hype around the estimation on the current size of the DIY population.
However, it is also true that findings on the perceived effects of tDCS appear to support more affirmative predictions on the future of the DIY use of tDCS. A total of 54 (44%) survey respondents reported that their use of tDCS was quite successful and satisfactory, and 111 respondents (92%) replied that they would continue using tDCS in the future. Also, 56 respondents (46%) are willing to recommend tDCS to family members or friends, who have the same aim(s) or condition(s) as they do.
Interviewees expressed conflicting views on the future of the DIY use of tDCS. Some argued that unless more robust evidence on the effects of tDCS emerges, tDCS would not continue attracting new users, and will disappear eventually. In contrast, others who strongly believed in the potential of tDCS as treatment and cognitive enhancement predicted substantial increase in the DIY tDCS user population.
These somewhat contradictory findings suggest that there are a lot of ambiguities and mistaken assumptions about the use of tDCS. In general, it can be said that at this stage, DIY use of tDCS is not currently widespread, that it does not seem to pose an imminent risk or danger to the public, and that there seems to be only a remote possibility of a dramatic increase of DIY use of tDCS in the near future. Nonetheless, more systematic and detailed investigations on the current state and prospect of DIY tDCS are required so that regulatory agencies can carefully examine the need for and the appropriate timing of potential regulatory interventions.
Mixed use of tDCS for treatment and cognitive enhancement
As discussed above, there is no regulation on therapeutic use of tDCS and devices intended for cognitive enhancement are not covered by any current regulations in the USA97 Considering possible regulatory options, Maslen et al. have expressed concerns about establishing a new separate regulatory regime for cognitive enhancement devices different from the current regime for medical devices.98 One of the reasons for their concern is that it is hard to categorically distinguish cognitive enhancement devices from medical devices.
Most of the current enhancing technologies, including tDCS, were originally developed for therapeutic purposes, such as treatments for illness or age-related mental deterioration, but the uses of these technologies have been subsequently expanded for enhancements.99 In other words, ‘[t]he very same device may be used both for therapeutic and enhancement purposes, in some cases using similar parameters’.100 The types and levels of risks that are involved with therapeutic and enhancement use of a device also may not be meaningfully different.101 In addition, some scholars have argued that there is no philosophical difference between treatment and enhancement—‘[b]oth therapy and enhancement aim to improve a human being's biology and/or psychology’.102
This study provides preliminary empirical support to the claim that the practical distinction between medical devices and enhancements can be muddy. The survey results showed the mixed and overlapping use of tDCS—13 (11%) respondents use tDCS for treatment of medical conditions, 71 (59%) respondents for cognitive enhancement, and 29 (24%) respondents for both cognitive enhancement and treatment. This suggests that if a regulatory agency decides to regulate tDCS either as a medical device or as a cognitive enhancement only, it will turn out to be an essentially underinclusive policy.
Existence of an active self-regulating system in the DIY tDCS user community
According to the survey results, DIY users claim to conform very well to safety guidelines for the current density (current dose divided by electrode size), and the total current dosage (current density multiplied by the duration of stimulation) set by previous clinical studies. The analysis on the web postings in subreddit tDCS demonstrated that DIY users are not only concerned about the safety of their own stimulation protocols but also cared about other users’ practice of tDCS. The general safety warnings by the users and the feedback process to verify the safety of individual DIY circuit show the existence of an active self-regulating system in this online forum.
According to previous literatures on self-regulation, self-regulation and state regulation can be ‘intertwined and complement each other’.103 The self-regulating system in the DIY tDCS community suggests that it may be possible to build a partnership between the DIY community and regulatory agencies to oversee the use of tDCS. In addition, the fact that many users consult website postings to get information on the three main elements (dose of current, size of electrodes, and duration of stimulation) of stimulation protocol reflects the users’ reliance on web postings to self-experiment with tDCS. Thus, incorporating this bottom-up self-regulating system into a top-down state regulatory framework can increase the efficiency of the framework by self-motivating DIY users to conform to the regulation and allowing the framework to more flexibly respond to unforeseen circumstances.
Fitz and Reiner claimed that regulatory approach taken to DIY by synthetic biologists (DIY BIO) could be a good example of a potential regulatory option for DIY tDCS.104 Synthetic biology is ‘the design and construction of new biological parts, devices, and systems, and the re-design of existing, natural biological systems for useful purposes’.105 In this case, the National Science Advisory Board for Biosecurity chose ‘open communication and education’ through public events and conferences instead of traditional regulatory practice to develop a culture of responsibility by DIY BIO practitioners.106
Fitz and Reiner acknowledged that compared to DIY BIO, the costs of practicing DIY tDCS are relatively low or sometimes even negligible, and thus, the risks associated with DIY tDCS are greater than those of DIY BIO.107 However, they argued that the new regulatory approach for DIY BIO can be a practical option at least for the interim period until a regulatory agency comes up with a comprehensive regime for tDCS.
The existence of the self-regulating system demonstrated in this study may provide support to Fitz and Reiner's argument for the new regulatory approach. With voluntary participation and oversight by DIY tDCS users, open communication and education can be a more viable and effective scheme for developing the culture of safe and responsible use.
However, it is noteworthy that recently, there has been a demographic shift within the DIY tDCS user community. The interviews with the power users of subreddit tDCS revealed that when this online forum was first established two years ago, most people in this forum had a background in engineering or did their ‘homework’ on the effects and risks of tDCS by researching previous clinical studies on tDCS before their self-experiments. The self-regulating body was comprised of these people, and they were ‘extremely helpful in giving real advices’.108 However, the recent release of ‘out of the box’ tDCS devices, such as foc.us, has increased interest in tDCS among the general public, and now there is a growing population of end users who do not know how tDCS circuits work or do not care to thoroughly investigate the effects and risks of tDCS.
The increase in the size of the DIY tDCS user community—particularly the influx of naïve end users—may undermine the vitality of the self-regulating system in subreddit tDCS. If the regulatory agency wants to incorporate the self-regulating system into any future regulatory regime for tDCS, it should take into account the potential impacts of this demographic shift in the community on the self-regulating system.
Desire for official guidelines and concern about government regulation of tDCS
A total of 97 out of 121 survey respondents (80%) reported that they had concerns about the DIY use of tDCS. These respondents are mostly worried about long-term impairment on the brain and the use of inaccurate placement of electrodes. Also, the same number of respondents answered that it would be helpful to have official guidelines from government agency or experts (researchers or physicians) for the DIY use of tDCS. In other words, it can be said that DIY users are waiting for the open communication and education by the authorities proposed in Fitz and Reiner's article to ensure the safety of their self-experiments with tDCS.
In addition, consistent with the survey results, all of the interviewed power user concurred that the issue where guidelines from the government or experts are most needed is placement of electrodes. As discussed above, locating the electrodes on the scalp to target a specific underlying brain region can be not only confusing but also very risky for lay users. Therefore, in designing a guideline or education program for DIY use of tDCS, the regulatory agency or expert groups may want to focus more on how to guide the placement of electrodes rather than other issues such as size of current or size of electrodes, for which there are already relatively clear safety standards.
In contrast to their desire for official guidelines, the survey respondents took a very negative stance toward potential government regulation. Some of the survey respondents’ answers showed that DIY users would practice tDCS regardless of the government regulations, to wit: ‘levying onerous requirements on DIY users may drive the work further underground to the benefit of none’.109 The significant financial advantages of DIY tDCS compared to other treatments for chronic medical conditions seem to make this scenario more plausible.
This raises a question whether it is legally or practically possible to enforce regulations on tDCS devices. tDCS devices may fall under the definition of medical ‘devices’ provided in the Federal Food, Drug, and Cosmetics Act. However, for the FDA to intervene and take enforcement actions, there need to be ‘prohibited acts’, such as the introduction of adulterated or misbranded drugs or devices into interstate commerce. Building and using a tDCS device only for oneself does not seem to constitute any of the prohibited acts listed in the Act.110
Moreover, even if the FDA may legally be able to outlaw the use of home-built tDCS devices through the broad interpretation or amendment of the Act, how could it enforce such a ban, considering the ease and low cost of making such devices and the difficulty of detecting them, especially considering the limited resources that the FDA has.
In addition, regulations of marketing and sales of ‘out of the box’ tDCS devices that would make device manufacturers subject to rigorous supervision, would likely lead to cost increases for the manufacturers that could increase the appeal of home-made tDCS devices. For example, arguing that enhancement devices in European Union should be regulated through extending the existing legislations for medical devices, Maslen et al. introduced a stringent supplementary requirement for device manufacturers. According to their proposal, manufacturers are required to provide ‘transparent, detailed, evidence-based information pertaining to the mechanisms, risks and effects that might be construed as benefits of the devices’111. ‘Providing such detailed information is currently not compulsory’.112 This demanding requirement will inflict additional costs on the manufacturers and eventually, the consumers.
However, given the ease of building tDCS devices and the DIY community's strong preference for low-cost devices, increasing the costs of devices may lead to uncontrollable self-manufacturer of the devices, causing users to hide out of reach of the FDA. Thus, further investigation is needed to analyse whether, in practice, the FDA intervention and supervision can effectively ensure the safety and responsible use of tDCS devices.
CONCLUSION: SUMMARY AND FUTURE DIRECTIONS
Direct brain enhancement is not an imaginary story that exists only in the remote future. One of the most promising enhancement technologies is tDCS. It was originally developed for treatment for medical conditions, such as depression, but clinical studies have also reported that it can improve various cognitive abilities among healthy people.
This study is the first empirical attempt to investigate the DIY tDCS user community. A questionnaire survey of DIY users, interviews with some active power users, and a content analysis of web postings on tDCS showed distinctive demographic characteristics of the DIY users, ambiguities and mistaken assumptions around the current state and future prospects of the DIY use of tDCS, mixed use of tDCS for both treatment and cognitive enhancement, the existence of an active self-regulating system in the community, and users’ demands for official guidelines and their concerns about government regulations on tDCS.
Even with the limitation of a small non-random sample, the findings of this study provide a preliminary but useful empirical illustration of the DIY tDCS user community, and increase our current understanding of the practice of DIY tDCS. However, more detailed subsequent studies are required to determine the need for and the timing of government interventions and to assess the strength and weakness of regulatory options proposed by researchers.
Furthermore, although this study just focused on one type of human biological cognitive enhancement—tDCS—there are numerous other enhancements, such as drugs, biologics, and dietary supplements, which could be used to improve the function of human bodies or brains.113 As discussed above, there have been growing discussions on whether and how to regulate these enhancements, including off-label uses of FDA-approved drugs and devices for enhancement purposes. However, empirical understanding on the use of these enhancements is incomplete, as in the case of tDCS. Owing to advances in biomedical science and increasing interest in human biological enhancements, these enhancements will be more available and accessible to the general public. Thus, for more meaningful and informed discussions of potential regulation, future studies on the actual practices of various human biological enhancements are required to clarify critical issues and factual assumptions regarding the use of these enhancements.
Appendix A: Survey Questionnaire
Appendix B: Coding Protocol for the Content Analysis
| MAIN THEMES OF THE POSTINGS | ||
| Name of Code | Detailed Classification under the Code | |
| 1 | Building and Operation of the tDCS device | 1.) Questions/reports on the DIY circuit |
| 2.) Questions/reports on electronic components of tDCS, such as electrodes | ||
| 3.) Questions on the stimulation protocol including duration of sessions, current size, and electrode size | ||
| 4.) Questions on the location of electrodes | ||
| 5.) Questions on the frequency of stimulation | ||
| 6.) Reports on the experience of mistakes and misuse of the device | ||
| 2 | Marketed tDCS device | 1.) Questions on ‘out of the box’ tDCS devices (asking recommendations) |
| 2.) Questions/reports on the operation of ‘out of the box’ tDCS devices | ||
| 3.) Reports on the defects in the product, delivery or service issues | ||
| 3 | Effects and Side Effects of the tDCS device | 1.) Questions/reports on the (perceived) effects and side effects of tDCS |
| 2.) Questions on the available cognitive tests to assess the effects of tDCS | ||
| 4 | References | Internet links to research paper, newspaper articles, videos, or blogs on tDCS and other brain stimulation technologies |
| 5 | Others | |
| SPECIFIC ISSUES ADDITIONALLY MENTIONED IN THE POSTINGS AND THE COMMENTS TO THE POSTINGS | ||
| Name of Code | Examples of Postings Come under the Code | |
| 7 | Aims (purposes) of using the tDCS device | 1.) Statements on the use of the tDCS device for treatment |
| 2.) Statements on the use of the tDCS device for cognitive enhancement | ||
| 8 | Safety warnings | General comments on the safety of the use of DIY tDCS by users |
Appendix C: Number of Users Per Number of Postings and Comments in www.reddit.com/r/tDCS
Appendix D: Period of Use among the Regular Users
Appendix E: Education Level of Respondents
Appendix F: Occupation of Respondents
Appendix G: Annual Income Level of Respondents
Appendix H: First Encounter of tDCS
Appendix I: Source of Information that DIY Users Consult for Determining the Three Factors
Appendix J: Perceived Effects of tDCS
Appendix K: Degree of Willingness to Recommend tDCS to Family Members or Friends
Appendix L: Types of Side Effects
Appendix M: Average Severity of Side Effects
Footnotes
*Anita Jwa is a J.S.D. candidate at Stanford University Law School. She holds an LL.B. in Law and an M.A. in Evolutionary Anthropology both from Seoul National University in Korea. She also earned a J.D. at Vanderbilt Law School and a J.S.M. at Stanford University Law School and worked as a law clerk at Supreme Court of Korea.
Henry T. Greely, Enhancing Brains: What Are We Afraid of?, Cerebrum, July 14, 2010, http://dana.org/news/cerebrum/detail.aspx?id=28786 (accessed Mar. 24, 2014).
Id.
Henry T. Greely, Law and the Revolution in Neuroscience, 42 Akron L. Rev. 687 (2009).
Greely, supra note 1; Greely, supra note 3; Henry T. Greely, Direct Brain Intervention to “treat” Disfavored Human Behaviors: Ethical and Social Issues, 91 Clin. Pharmacol. & Ther. 163 (2012); Roy Hamilton et al., Rethinking the Thinking Cap: Ethics of Neural Enhancement Using Noninvasive Brain Stimulation, 76 Neurology 187 (2011).
Greely, supra note 1.
Kate E. Hoy et al., Testing the limits: Investigating the Effect of tDCS Does on Working Memory Enhancement in Healthy Controls, 51 Neuropsychologia 1777 (2013).
Andre Brunoni et al., Clinical Research with Transcranial Direct Current Stimulation (tDCS): Challenges and Future Directions, 5 Brain Stimul. 175 (2012); Michael A. Nitsche & Walter Paulus, Transcranial Direct Current Stimulation—Update 2011, 29 Restor. Neurol. & Neurosci. 463 (2011).
Alberto Priori, Brain Polarization in Humans: A Reappraisal of an Old Tool for Prolonged Non-invasive Modulation of Brain Activity, 114 Clin. Neurophysiol. 589 (2003).
Id.
See eg Felipe Fregni et al., Treatment of Major Depression with Transcranial Direct Current Stimulation, 8 Bipolar Disord. 203 (2008); Felipe Fregni et al., A Sham-controlled, Phrase II Trial of Transcranial Direct Current Simulation for the Treatment of Central Pain in Traumatic Spinal Cord Injury, 122 Pain 197 (2006); Julie Baker et al., Using Transcranial Direct Current Stimulation (tDCS) to Treat Stroke Patients with Aphasia, 41 Stroke 1229 (2010).
See eg Lars A. Ross et al., Improved Proper Name Recall by Electrical Stimulation of the Anterior Temporal Lobes, 48 Neuropsychologia 3671, (2010); Meinou H. de Vries et al., Electrical Stimulation of Broca's Area Enhances Implicit Learning of an Artificial Grammar, 22 J. Cognitive Neurosci.2427 (2010); Min-Fang Kuo & Michael A. Nitsche, Effects of Transcranial Electrical Stimulation on Cognition, 43 Clin. EEG & Neurosci.192,195 (2012); Nadia Bolognini et al., Brain Polarization of Parietal Cortex Augments Training-Induced Improvement of Visual Exploratory and Attentional Skills, 1349 Brain Res. 76 (2010); Paul G. Mulquiney et al., Improving Working Memory: Exploring the Effect of Transcranial Random Noise Stimulation and Transcranial Direct Current Stimulation on the Dorsolateral Prefrontal Cortex, 122 Clin. Neruophysiol. 2384 (2011).
de Vries et al., supra note 11.
Kuo & Nitsche, supra note 11.
Ross et al., supra note 11.
Vincent P. Clark et al., tDCS Guided Using fMRI Significantly Accelerates Learning to Identify Concealed Objects, 59 Neuroimage 117 (2012).
Kate Murphy, Jump-Starter Kits for the Mind, New York Times, Oct. 28, 2013, at D3. See also Peter Murray, Does Passing A Small Current Through Your Brain Really Make You Smarter?, Singularity Hub, Jan. 14, 2013, http://singularityhub.com/2013/01/14/does-passing-a-small-current-through-your-brain-really-make-you-smarter/ (accessed Mar. 24, 2014); Greg Miller, The Unfinished Science Behind the New Waive of Electrical Brain Stimulation, Wired, May 5, 2014, http://www.wired.com/2014/05/brain-stimulation-science/ (accessed Mar. 24, 2014); Greg Miller, Inside the Strange New World of Brain Stimulation, Wired, May 5, 2014, http://www.wired.com/2014/05/diy-brain-stimulation/ (accessed Mar. 24, 2014).
Hamilton et al., supra note 4, at 188.
Clinical Trials. Gov—tDCS, http://clinicaltrials.gov/ct2/results/map?term=tdcs (accessed Jan. 17, 2014).
Nicholas S. Fitz & Peter B. Reiner, The Challenge of Crafting Policy for Do-It-Yourself Brain Stimulation, J. Med. Ethics, DOI:10.1136/medethics-2013–101458 (2013); Meenakshi B. Iyer et al., Safety and Cognitive Effect of Frontal DC Brain Polarization in Healthy Individuals, 64 Neurology 872 (2005).
TCT, www.trans-cranial.com (accessed Mar. 24, 2014); FOC.US, www.foc.us (accessed Mar. 24, 2014); tDCS Device Kit, tdcsdevicekit.com (accessed Mar. 24, 2014); Mind Alive Inc., www.mindalive.com (accessed Mar. 24, 2014); Neuroconn, www.neuroconn.de (accessed Mar. 24, 2014).
Fitz & Reiner, supra note 19; Hamilton et al., supra note 4; Jan-Hendrik Heinrichs, The Promises and Perils of Non-invasive Brain Stimulation, 35 Int'l. J. L. & Psychiatry 121(2012); Roi Cohen Kadosh et al., The Neuroethics of Non-Invasive Brain Stimulation, 22 Current Biol. 108 (2012).
Fitz & Reiner, supra note 19.
Id.
Id.
Teresa Lucalano & Roi Cohen Kadosh, The Mental Cost of Cognitive Enhancement, 33 J. Neurosci. 4482 (2013).
US Federal Food and Drug Administration, Is The Product A Medical Device?, http://www.fda.gov/medicaldevices/deviceregulationandguidance/overview/classifyyourdevice/ucm051512.htm (accessed Mar. 24, 2014).
Federal Food, Drug, and Cosmetics Act § 201(h), 21 U.S.C. § 321(h) (2012).
Judith A. Johnson, Cong. Research Serv., R42130, FDA Regulation of Medical Devices (2012) https://www.fas.org/sgp/crs/misc/R42130.pdf (accessed Mar. 24, 2014); 21 C.F.R. parts 862, 892 (2014).
Id. at 4; Federal Food, Drug, and Cosmetics Act § 513, 21 U.S.C. § 360(c) (2012).
Id. at 3.
Id. at Summary; 21 C.F.R. § 814 (2014).
Id.
Id.; Federal Food, Drug, and Cosmetics Act §510(k), 21 U.S.C. § 360(k) (2012).
Johnson, supra note 28, at 8.
Id. at 13.
21 C.F.R. § 882.5805 (2014).
21 C.F.R. § 882.5800 (2014).
US Food and Drug Administration, Petitions to request change in classification for cranial electrotherapy stimulators, (FDA executive summary, prepared for the Feb. 10, 2012 meeting of the Neurologic Devices Panel), 2012, http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/NeurologicalDevicesPanel/UCM290787.pdf (accessed Mar. 24, 2014).
Beth Israel Deaconess Medical Center, TMS and tDCS Treatments, http://www.bidmc.org/Centers-and-Departments/Departments/Neurology/Noninvasive-Brain-Stimulation/Patient-Care/TMS-and-tDCS-Treatments.aspx (accessed Mar. 24, 2014); The Brain Stimulation Clinic, Medical Doctor of the Brain Stimulation Clinic, http://www.transcranialbrainstimulation.com/doctor-fugedy (accessed Mar. 24, 2014); 21 C.F.R. 890.5525 (2014).
Hannah Maslen et al., Do-It-Yourself Brain Stimulation: A Regulatory Model, J. Med. Ethics, DOI:10.1136/medethics-2013–101692 (2013).
Fitz & Reiner, supra note 19.
Murphy, supra note 16; FOC.US, www.foc.us (accessed Mar. 24, 2014).
Fitz & Reiner, supra note 19.
Id.
Maslen et al., supra note 40.
California Department of Public Health, CDPH Warns Consumers Not to Use TDCS Home Device Kit, June 28, 2013, http://www.cdph.ca.gov/Pages/NR13-029.aspx (accessed Mar. 24, 2014).
The survey and interview protocols of this study were approved by the Stanford Institutional Review Board. All participants provided informed consent—the participants were anonymous and did not receive any payment for their participation.
The total number of completed responses is 125. However, three respondents stated in the questionnaire that they actually have never used the tDCS device. In addition, one respondent answered the questionnaire on behalf of his four-year-old daughter who has been undergoing tDCS treatment by a physician for her motoric alalia and dyslexia. These four responses were excluded in the final data analysis.
Kimberly A. Neuendorf, The Content Analysis Guidebook 102 (2002).
One of the five power users did not answer to the interview request.
The Webmaster of diytdcs is not a tDCS ‘user’ in the strict sense, since he has never used the tDCS device for himself although he has compiled a well-known tDCS research database at diytdcs and advised many DIY users concerning the design and use of tDCS at subreddit tDCS since the establishment of the forum. However, this study interviewed the Webmaster as a ‘power user’ expecting that he is more knowledgeable about the previous and current trends in the DIY tDCS user community than average DIY tDCS users.
All the interviewees agreed to be identified as described in this paragraph.
Interview with the Webmaster of diytdcs.
Id.
Two users (2%) preferred not to disclose their gender.
More detailed geographic distribution of the respondents was as follows (the number in the parenthesis indicates the number of respondents): Australia (3), Brazil (1), Canada (8), Estonia (1), France (1), Germany (3), Hong Kong (1), Hungary (1), India (3), Israel (1), Italy (2), Netherlands (1), Norway (1), Philippines (1), Poland (1), Russian Federation (1), Singapore (1), Spain (1), Sweden (1), the UK (5), the USA (81), and Viet Nam (1).
The number of respondents who answered that they are using tDCS for treatment of medical condition was 12, and there are nine respondents who answered ‘other.’ In case where a respondent chose ‘other’, he/she was asked to give more detailed information. However, one of the respondents who answered ‘other’ provided that he is using tDCS for ‘sleep and depression.’ This study counted this respondent in the total number of respondents who are using tDCS for treatment. It should be noted that some of questions, which will be analysed below, were only displayed to the respondents who answered that they are using tDCS for treatment or for treatment and enhancement. This respondent did not take these questions and thus, was not included in the analysis below.
‘Other’ purposes that do not fall under treatment or enhancement were curiosity, experiment (without any other motive), or research.
The respondents were allowed to choose more than one cognitive enhancement purpose.
This question was only displayed to 41 respondents who answered that they are using tDCS for treatment or both for treatment and enhancement. (See supra note 57). Also, respondents were allowed to choose more than one medical condition.
Fitz & Reiner, supra note 19.
This question was only displayed to 41 respondents who answered that they are using tDCS for treatment or both for treatment and enhancement. (See supra note 57).
However, although he did not scientifically control or test his experiences with tDCS treatment, the physician stated that he did not advise his patients to stop taking other medications and he believed that medicine like antidepressant actually ‘gives additional benefits’ combined with tDCS treatment. In addition, he suggested that some other medications interfered with the effects of tDCS but they did not eliminate the effects. Thus, to compensate, he recommended that his patients undergo more treatments at clinic or do longer protocols when they are self-treating at home. Also, the interviewee who is using tDCS for treatment of bipolar type II disorder reported that he thought that tDCS and psychoactive drugs are complementary treatments but their effects are not compounded—he claimed he was able to identify the effects of drugs and tDCS distinctively.
Five respondents answered ‘other’. Two of these respondents reported that they are using a device at their university and research institution.
Ohsnapitsnathan, Open Source tDCS Project, Reddit tDCS, Mar. 27, 2012, http://www.reddit.com/r/tDCS/comments/rgzv6/opensource_tdcs_project/ (accessed Mar. 24, 2014); NathanWW, OpenStim, Sourceforge, http://sourceforge.net/projects/openbrainstim/(accessed Jan. 29, 2013).
Shawn Nock, Two New Open tDCS Designs, Nocko.se, Aug. 8, 2012, https://nocko.se/2012/07/30/brain-zapping-is-fun/ (accessed Mar. 24, 2014); https://nocko.se/2012/08/08/opentdcs/ (accessed Mar. 24, 2014).
Interview with the moderator of subreddit tDCS.
Id.
Id.
eg 55tfg7879fe42e345, DIY tDCS Howto, Reddit tDCS, Mar.3, 2012, http://www.reddit.com/r/tDCS/comments/qn6s5/diy_tdcs_howto/ (accessed Mar. 24, 2014); Jessieheys, Simple DIY tDCS Device, Reddit tDCS, June 27, 2012, http://www.reddit.com/r/tDCS/comments/vpob3/simple_diy_tdcs_device/ (accessed Mar. 24, 2014).
In this question, respondents were allowed to choose more than one ‘out of the box’ tDCS device.
Five respondents who answered ‘other’ reported that they are using the device from tdcs-kit.com or soterixmedical.com/tdcs.
For the image of foc.us device, Foc.us, http://www.foc.us (accessed Mar. 24, 2014).
Interview with the moderator of subreddit tDCS.
Id.
Brunoni et al., supra note 7; Priori, supra note 8; Csaba Poreisz et al., Safety Aspects of Transcranial Direct Current Stimulation Concerning Healthy Subjects and Patients, 72 Brain Res. Bull. 208 (2007).
Brunoni et al., supra note 7, at 183; Here, the total current dose means the amount of current delivered to the electrodes which lie on the outside of the skull. A subset—an unknown subset in most cases—then passes through the skin and the skull to reach the brain.
Id.
In this question, respondents were allowed to choose more than one source of information.
See eg Asli Demirtas-Tatlidede et al., Can Noninvasive Brain Stimulation Enhance Cognition in Neuropsychiatric Disorders?, 64 Neuropharmacology 566, 570 (2013).
See eg Elokane, Safety of Frequent tDCS Use, Reddit tDCS, Jan. 18, 2013, http://www.reddit.com/r/tDCS/comments/16u55u/safety_of_frequent_tdcs_use/ (accessed Mar. 24, 2014); Bigbacondude, tDCS Stimulation Duration/Timings Question, Reddit tDCS, Aug. 17, 2013, http://www.reddit.com/r/tDCS/comments/1kkp8n/tdcs_stimulation_durationtimings_question/ (accessed Mar. 24, 2014); Livefromkoyto, How Often Is It Safe to Use tDCS?, Reddit tDCS, Aug. 19, 2013, http://www.reddit.com/r/tDCS/comments/1kov8n/how_often_is_it_safe_to_use_tdcs/ (accessed Mar. 24, 2014); Satisfyinghump, What's the Most Times You Can Do Individual tDCS, Reddit tDCS, Oct. 5, 2013, http://www.reddit.com/r/tDCS/comments/1nskpw/whats_the_most_times_you_can_do_individual_tdcs/ (accessed Mar. 24, 2014); Mishefe, Have We Learned Anything about Max Daily Usage, Reddit tDCS, Oct. 25, 2013, http://www.reddit.com/r/tDCS/comments/1p81pm/have_we_learned_anything_new_about_max_daily_usage/ (accessed Mar. 24, 2014).
The question on the frequency of use was only displayed to the 62 respondents who are regular and continuous users. However, this question did not force respondents to answer, and 56 respondents completed this question.
Keneticforce, tDCS Home Built: Great Results ... Alarming Negative Side Effects, Reddit tDCS, June 9, 2013, http://www.reddit.com/r/tDCS/comments/1fzb9c/tdcs_home_built_great_results_alarming_negative/ (accessed Mar. 24, 2014).
Lumosity, http://www.lumosity.com (accessed Mar. 24, 2014); Brain Workshop—A Dual N-Back Game, http://brainworkshop.sourceforge.net (accessed Mar. 24, 2014); Cambridge Brain Sciences, http://www.cambridgebrainsciences.com/challenge/introduction (accessed Mar. 24, 2014).
See supra note 83.
Torvaun, So, It's a Few Hours Past My First Montage, Reddit tDCS, Jul. 23, 2013, http://www.reddit.com/r/tDCS/comments/1ixpdx/so_its_a_few_hours_past_my_first_montage/ (accessed Mar. 24, 2014).
This list of side effects used in the multiple-choice survey question was adopted from Poreisz et al.'s study on the safety of tDCS. Poreisz et al., supra note 76; In this question, respondents were allowed to choose more than one side effects.
Abraham P. Arul-Anandam, Colleen Loo & Perminder Sachdev, Transcranial Direct Current Stimulation—What Is the Evidence for Its Efficacy and Safety?, F1000 Med. Rep., DOI:10.3410/M1–58 (2009); Iyer et al., supra note 19; Poreisz et al., supra note 76.
In this question, respondents were allowed to choose more than one side effect.
In this question, respondents were allowed to choose more than one concern on the DIY use of tDCS.
See eg OmegaRa, My DIY Unit, Reddit tDCS, Dec. 13, 2013, http://www.reddit.com/r/tDCS/comments/1su22g/my_diy_unit/ (accessed Mar. 24, 2014); Rpxo, Check Out My DIY; LM334Z Design, Reddit tDCS, Jan. 2, 2014, http://www.reddit.com/r/tDCS/comments/1u95m3/check_out_my_diy_lm334z_design/ (accessed Mar. 24, 2014); OJandToothpaste, Will My DIY Setup Melt My Brain, Reddit tDCS, Aug. 7, 2013, http://www.reddit.com/r/tDCS/comments/1jvc5j/will_my_diy_setup_melt_my_brain/ (accessed Mar. 24, 2014).
Interview with a user who has uploaded popular self-experimentation videos on his YouTube Channel.
Fitz & Reiner, supra note 19.
Id.
Interview with the Webmaster of diytdcs.
Interview with the moderator of subreddit tDCS.
The regulatory regime of European Union also does not cover therapeutic tDCS and devices for cognitive enhancement.
Hannah Maslen et al., The Regulation of Cognitive Enhancement Devices: Extending the Medical Model, 1 J. L. & Biosci. 68, 79 (2014).
Greely, supra note 1.
Maslen et al., supra note 98.
Id.
Id.
Jean P. Mifsud Bonnici, Self-Regulation in Cyber Space 6 (2008).
Fitz & Reiner, supra note 19
U.S.C.
Syntheticbiology, http://syntheticbiology.org (accessed Mar. 24, 2014).
Id.; National Science Advisory Board for Biosecurity, Strategies to educate amateur biologists and scientists in non-life science disciplines about dual use research in the life sciences, June 2011, http://osp.od.nih.gov/sites/default/files/resources/FinalNSABBReport-AmateurBiologist-NonlifeScientists_June-2011_0.pdf (accessed Mar. 24, 2014).
Fitz & Reiner, supra note 19.
Interview with a user who has uploaded popular self-experimentation videos on his YouTube Channel.
Id.
Federal Food, Drug, and Cosmetics Act § 301, 21 U.S.C. § 331 (2012).
Maslen et al., supra note 98, at 88.
Id.
Henry T. Greely, Of Nails and Hammers: Human Biological Enhancement and U.S. Policy Tools, in Enhancing Human Capabilities 503 (Julian Lucalano et al. eds, 2011).