Fig. 2.
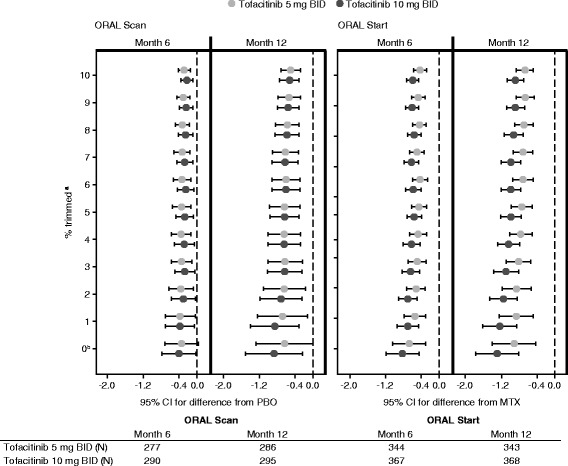
Trimmed analysis of differences from comparator in mTSS at month 6 and month 12 in ORAL Scan and ORAL Start. 0 % trimming represents the primary analysis. aPercentage of data excluded; LS mean differences from PBO (ORAL Scan) or MTX (ORAL Start) with 95 % CIs of each tofacitinib group vs comparator are presented; a CI that does not contain 0 indicates that the difference is statistically significant (p < 0.05). BID twice daily, CI confidence interval, LS least squares, mTSS van der Heijde modified total Sharp score, MTX methotrexate, N number of patients eligible for analysis, PBO placebo
