Abstract
Objective
Brain regulation of glucose homeostasis is sexually dimorphic; however, the impact sex hormones have on specific neuronal populations within the ventromedial hypothalamic nucleus (VMN), a metabolically sensitive brain region, has yet to be fully characterized. Glucose-excited (GE) and -inhibited (GI) neurons are located throughout the VMN and may play a critical role in glucose and energy homeostasis. Within the ventrolateral portion of the VMN (VL-VMN), glucose sensing neurons and estrogen receptor (ER) distributions overlap. We therefore tested the hypothesis that VL-VMN glucose sensing neurons were sexually dimorphic and regulated by 17β-estradiol (17βE).
Methods
Electrophysiological recordings of VL-VMN glucose sensing neurons in brain slices isolated from age- and weight-matched female and male mice were performed in the presence and absence of 17βE.
Results
We found a new class of VL-VMN GI neurons whose response to low glucose was transient despite continued exposure to low glucose. Heretofore, we refer to these newly identified VL-VMN GI neurons as ‘adapting’ or AdGI neurons. We found a sexual dimorphic response to low glucose, with male nonadapting GI neurons, but not AdGI neurons, responding more robustly to low glucose than those from females. 17βE blunted the response of both nonadapting GI and AdGI neurons to low glucose in both males and females, which was mediated by activation of estrogen receptor β and inhibition of AMP-activated kinase. In contrast, 17βE had no impact on GE or non-glucose sensing neurons in either sex.
Conclusion
These data suggest sex differences and estrogenic regulation of VMN hypothalamic glucose sensing may contribute to the sexual dimorphism in glucose homeostasis.
Keywords: 17β-estradiol, AMP-activated kinase, Glucose excited neurons, Glucose inhibited neurons, Ventromedial hypothalamic nucleus, Sexual dimorphism
Abbreviations: 17βE, 17β-estradiol; AICAR, aminoimidazole-4-carboxamide-1-β-d-ribofuranoside; AMPK, AMP-activated protein kinase; ARC, arcuate nucleus; BSA-17βE, bovine serum albumin-conjugated 17βE; CC, compound C; ER, estrogen receptor; GABA, γ-aminobutyric acid; GE, glucose-excited; GI, glucose-inhibited; HRP, horse radish peroxidase; IR, input resistance; MPP, methylphenolpyrazole; NGS, non-glucose sensing; PHTPP, phenyltrifluoromethylpyrazolophenol; POMC, pro-opiomelanocortin; PVDF, polyvinylidene difluoride; SF-1, steroidogenic factor; TTX, tetrodotoxin; VMH, ventromedial hypothalamus; VL-VMN, ventrolateral VMN; Vm, membrane potential; VMN, ventromedial hypothalamic nucleus
Highlights
-
•
Hypothalamic glucose sensitivity is sexually dimorphic.
-
•
17βE blunts activation of glucose inhibited neurons in low glucose.
-
•
Estrogen regulation of glucose sensing may mediate sexual dimorphisms in glucose homeostasis.
1. Introduction
Estrogens, particularly hypothalamic 17β-estradiol (17βE), play an important role in energy and glucose homeostasis [1]. The ventromedial hypothalamic nucleus (VMN) is a critical site of 17βE action in energy balance [2]. Deletion of estrogen receptor alpha (ERα) in VMN steroidogenic factor (SF-1) neurons leads to alterations in metabolism resulting in visceral adiposity and infertility which is observed only in females, suggesting a sexually dimorphic impact of estrogenic signaling within the VMN to regulate metabolism [3]. The VMN is also important for hypoglycemia detection and initiation of the sympathoadrenal and hormonal counterregulatory responses, which restore euglycemia [4], [5]. There is a sexual dimorphic response to hypoglycemia, with females exhibiting a reduction in sympathetic drive [6], glucagon [7] and epinephrine secretion [8], [9], and hepatic glucose output [10] when compared to males. These observations suggest that VMN neurons may play a role in the sex differences in glucose and energy homeostasis; however, the mechanism by which they do so is not currently known.
The VMN contains non-glucose sensing (NGS) as well as glucose sensing neurons, which increase (glucose-excited, GE) or decrease (glucose-inhibited, GI) their activity as extracellular glucose levels increase. Glucose closes ATP-sensitive K+ channels leading to activation of VMN GE neurons. In contrast, glucose inhibits the fuel sensor, AMP-activated protein kinase (AMPK) leading to Cl− channel opening and inhibition of VMN GI neurons [11], [12]. Our lab has previously shown that VMN GI neurons are important for hypoglycemia detection and counterregulation [12], [13], [14]. Moreover, the hormones insulin and leptin, which signal energy sufficiency, inhibit VMN GE and GI neurons and blunt their response to low glucose [15], [16]. VMN GE neurons, like estrogen receptors (ERs), are concentrated in the ventrolateral (VL)-VMN, while VMN GI neurons can be found in all subnuclei within the VMN (including the VL-VMN) [11], [15], [17]. Therefore, VL-VMN estrogen receptors, which are critical for regulating energy sufficiency, may also be critical in mediating glucose homeostasis.
ER expression and the distribution of glucose sensing neurons overlap within the VL-VMN [11], [15], [17]. Moreover, VL-VMN ER expression is sexually dimorphic [18]. Thus, we hypothesized that the glucose sensitivity of VL-VMN glucose sensing neurons may also be sexually dimorphic. We further hypothesized that since the effects of 17βE on energy homeostasis overlap to some degree with those of insulin and leptin, 17βE would similarly regulate the activity and/or glucose sensitivity of VL-VMN glucose sensing neurons. These hypotheses were tested using electrophysiological recordings of VL-VMN glucose sensing neurons in brain slices isolated from 3 to 4 week old male and female mice. Interestingly, in the course of our investigation we found a novel class of VL-VMN GI neurons whose response to changes in extracellular glucose concentration was transient despite continued exposure to low glucose. These GI neurons will be referred to as ‘adapting’ or AdGI neurons in comparison to those whose activity is maintained in low glucose (nonadapting). We found that VL-VMN nonadapting GI, but not AdGI, neurons are sexually dimorphic. Furthermore, 17βE regulates the activity and/or glucose sensitivity of both VL-VMN nonadapting GI and AdGI neurons in males and females similarly. Our data suggest that VL-VMN nonadapting GI and AdGI neurons may contribute to the sex differences observed in energy and glucose homeostasis.
2. Material and methods
2.1. Animals and tissue preparation
Age and weight matched male and female C57Bl/6 mice were maintained on a 12:12 light:dark cycle with standard rodent chow and water ad libitum. Animal procedures were approved by the Institutional Animal Care and Use Committee at Rutgers, The State University of New Jersey, RBHS-New Jersey Medical School. The average age and weight of animals used in this study was ♂25.6 ± 0.4, ♀25.6 ± 0.2 days old and ♂14.0 ± 0.4, ♀13.3 ± 0.2 g, respectively. Female mice were only used prior to vaginal opening, a visual marker of pubertal transition [19]. This age range (3–4 weeks) was chosen in order to evaluate organizational and inherent sex differences of glucose sensing neurons. Following transcardial perfusion with 4 °C oxygenated (95%O2/5%CO2) perfusion buffer (composition in mM: 2.5 KCl, 7 MgCl2, 1.25 NaH2PO4, 28 NaHCO3, 0.5 CaCl2, 7 glucose, 1 ascorbate, 3 pyruvate; ∼300 mOsm, pH 7.4), brains were quickly removed and placed in 4 °C oxygenated perfusion buffer. The hypothalamus was dissected and 250–300 μm coronal slices were made on a vibratome (Vibroslice, Camden Instruments).
2.2. Electrophysiology
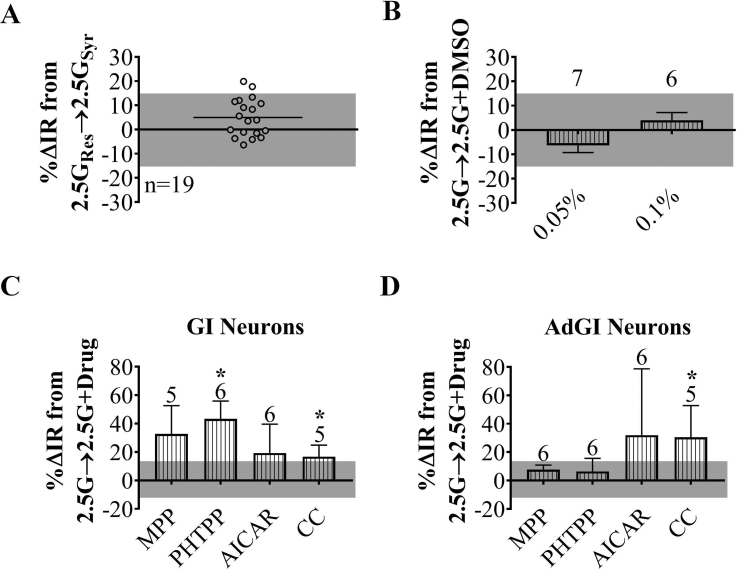
VMN containing slices (3 per animal) were maintained in oxygenated artificial cerebrospinal fluid (aCSF, in mM: 126 NaCl, 1.9 KCl, 1.2 KH2PO4, 26 NaHCO3, 2.5 glucose, 1.3 MgCl2, 2.4 CaCl2; ∼300 mOsm, pH 7.4) for at least one hour prior to recording. Standard whole-cell current clamp recordings were made as previously described [20]. Briefly, borosilicate pipettes (4–7 MΩ; Sutter Instruments) were filled with an intracellular solution containing (in mM): 128 K-gluconate, 10 KCl, 4 KOH, 10 HEPES, 4 MgCl2, 0.5 CaCl2, 5 EGTA, 2 Na2ATP, 0.4 Na2GTP; ∼300 mOsm, pH 7.2. Pipette solution junction potential was nulled prior to formation of a GΩ seal, membranes were ruptured with mild suction followed by whole cell capacitance compensation. VL-VMN neurons were identified according to the position of anatomical markers (i.e., 3rd ventricle, fornix) outlined in the mouse brain atlas [21]. Cells were deemed viable for recording if action potentials crossed 0 mV and access resistance was less than 30 MΩ following membrane rupturing. Input resistance (IR) was calculated from hyperpolarizing current pulses given every three seconds (500 ms; −10, −15 or −20 pA). IR reflects cell excitability and responsiveness to presynaptic input with a higher IR reflecting greater excitability. IR is a valid and useful index of glucose responses, because glucose modulates background or “leak” K+ or Cl− channels, which set membrane potential and overall cellular excitability [11]. IR is also independent of changes in action potentials, which may vary between slices depending on the presynaptic inputs remaining intact in any given slice. Slices were exposed to the various treatment solutions for ten minutes each based on previous studies showing this exposure time was sufficient to determine glucose and hormone responses in glucose sensing neurons [11], [15], [22], [23], [24], [25]. Current-voltage (I–V) relationships were determined following each treatment in I-clamp mode by injection of hyperpolarizing step pulses (500 ms duration). Step pulse protocols ranging from −160 pA to 0 pA were terminated once the membrane voltage response hyperpolarized to −100 mV. A response of ±15.6% change from baseline in IR (as determined in Supplemental Figure 1A), which reversed by >50% was used to distinguish NGS from glucose sensitive neurons and 17βE-sensitive from 17βE-insensitive neurons.
2.3. Slice treatments
Glucose sensing was evaluated in response to a glucose decrease from 2.5 mM to 0.1 mM. These glucose concentrations represent those typically seen in the brain during peripheral euglycemia and severe hypoglycemia, respectively [26], [27]. This maximal glucose decrease was used since attenuated responses to smaller glucose decreases might be below the limit of detection and lead to incorrect conclusions that glucose sensing neurons were absent or reduced in total number by sex or treatment. Similarly, for 17βE sensitivity experiments, slices were treated with a supraphysiological concentration (100 nM) [28] to help ensure that 17βE-mediated responses were above the limit of detection. For mechanistic studies, glucose was lowered in the presence and absence of 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; 0.5 mM) [15], Compound C (CC; 10 μM) [15], methylphenolpyrazole (MPP; 10 μM) [29], phenyltrifluoromethylpyrazolophenol (PHTPP; 1 μM) [30], tetrodotoxin (TTX, 500 nM) [22], or tolbutamide (100 μM) [25]. In some cases, glucose was lowered in the presence or absence of the above with and without 17βE (100 nM) or bovine serum albumin-conjugated 17βE (BSA-17βE; 100 nM) [31]. When necessary, DMSO was used as the vehicle for drug treatments, but the final DMSO concentration for all treatments was ≤0.1%. At this concentration, no significant changes in basal neuronal activity were observed (Supplemental Figure 1B). Supplemental Figure 1C and D shows the effects of the above agonists and antagonists individually in 2.5 mM glucose. To rule out the contribution of individual agonist or antagonist effects, we compared the effects of low glucose or 17βE in the presence of these pharmacological treatments relative to baseline (2.5 mM glucose) with the same pharmacological treatment. On average 4 neurons (approximately 1.5 h each) were recorded per slice, but no slice was exposed to 17βE for more than 30 min.
2.4. Immunoblotting
Due to the size of the mouse hypothalamus it was not possible to dissect only the VMN without including the adjacent ARC. Thus, when discussing the immunoblotting experiments, we will refer to the “VMH” as an indication that both VMN and ARC are included. This is opposed to electrophysiological studies in which VMN neurons were visualized for specific recording. After isolation, VMH containing slices were maintained in 2.5 mM glucose aCSF for 30 min and then transferred to either 2.5 or 0.1 mM glucose aCSF for 15 min. A separate set of slices were preincubated with 100 nM 17βE or 100 nM BSA-17βE (Steraloids 5:1 hemisuccinate) in 2.5 mM glucose aCSF for 15 min and then transferred to 0.1 mM glucose aCSF in the presence of 17βE or BSA-E for 15 min. BSA-17βE was prepared fresh daily and filtered through Amicon® Ultra Centrifugal Filter (30 K Nominal Molecular weight limit) devices (Millipore) to eliminate any unbound 17βE in the treatment solutions [31]. After each treatment, three VMH containing slices representing the 1) anterior, 2) medial, and 3) posterior VMH (based on the size and shape of the 3rd ventricle) were collected from different animals and pooled. The VMH excised and homogenized by sonication. Subsequently, cells were lysed and immunoblotted for phospho-Thr172 AMPKα, total AMPK, and β-actin, as previously described [32]. Briefly, 20–60 μg of protein was separated on 10% polyacrylamide gels and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% BSA and incubated with the primary and horse radish peroxidase (HRP) conjugated-secondary antibodies. Membranes were probed, stripped, and re-probed with primary antibodies in the following order: phospho-Thr172 AMPKα, total AMPK and β-actin. β-actin was used as a loading control, and treatment effects were normalized to β-actin and 2.5 mM glucose samples within each gel.
2.5. Data analysis & statistics
All data are expressed as average ± standard error. For electrophysiological recordings, treatment effects were quantified using the percent changes in input resistance (IR) and membrane potential (Vm) from that in 2.5 mM glucose. IR was calculated from the membrane voltage responses to hyperpolarizing pulses within the last minute of treatment application according to Ohm's law (R = V/I) where R is resistance, V is voltage, and I is current. For AdGI neurons, IR in 0.1 mM glucose was calculated for one minute once maximal activation of these neurons occurred (∼5–6 min after solution change). 17βE-sensitivity (in 2.5 mM glucose) was determined based on a threshold value established during control solution exchange experiments (Supplemental Figure 1). In these control experiments, we calculated ±2xSTD for percent change in IR observed by changing the source of the perfusion solution from the general reservoir to the treatment syringe while keeping the solution composition constant. Any cell displaying a percent change in IR ≥ ±2xSTD (±15.6%) was considered 17βE-sensitive. Glucose sensitivity was confirmed similarly. Current-voltage (I–V) relationships were fitted with linear best-fit lines. For immunoblotting studies, β-actin was used as a loading control, and treatment effects were normalized to 2.5 mM glucose samples within each gel.
Data was compared using paired or unpaired student t-tests and repeated or standard one-way ANOVA with Tukey posthoc tests or two-way ANOVA with Bonferonni posthoc tests as specified in figure legends. For t-tests, *p < 0.05, **p < 0.01, and ***p < 0.001. For ANOVAs, columns with different letters indicate significant differences.
3. Results
3.1. VL-VMN glucose sensing subtypes
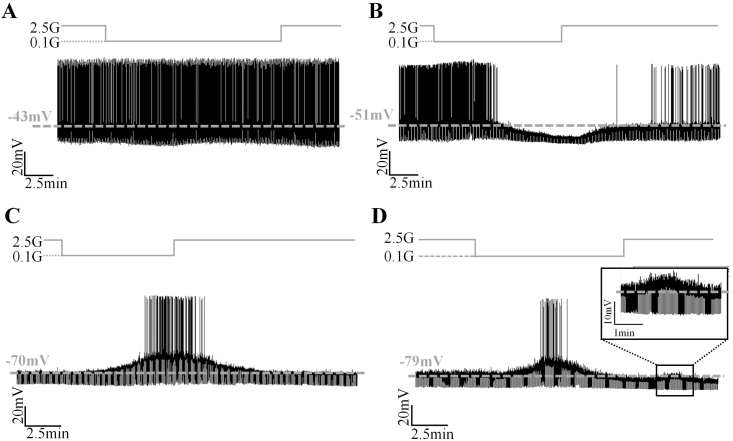
Within the VL-VMN, we identified four different neuronal populations with respect to glucose sensitivity: non-glucose sensing (NGS), glucose-excited (GE), nonadapting glucose-inhibited (GI) and adapting glucose-inhibited (AdGI; Figure 1). NGS neurons, by definition, did not respond to changing glucose levels (Figure 1A). GE, nonadapting GI, and AdGI neurons altered their activity in response to a decrease in extracellular glucose from 2.5 mM to 0.1 mM (Figure 1B–D). Table 1 describes the criteria used to define each neuronal type.
Figure 1.
VL-VMN glucose sensing neuron subtypes. (A–D) Representative consecutive whole cell current-clamp recordings of a female NGS, (A) GE, (B) nonadapting GI, (C) and adapting GI (D) neurons. Figure 1D Inset: Magnification of a secondary glucose response following solution change to 2.5 mM glucose. Glucose changes are schematically displayed above each recording; dashed grey line represents resting Vm. G: mM glucose, IR: input resistance, Vm: membrane potential.
Table 1.
Identification criteria and distribution of VL-VMN glucose sensing subtypes.
| Neuron type | Response (%Δ) to 0.1 mM glucose |
VL-VMN distribution, n (%) |
||||
|---|---|---|---|---|---|---|
| Vm | IR | Duration | Reversal | Males | Females | |
| NGS | No change | No change | NA | NA | 7 (17) | 60 (20) |
| GE | Hyperpolarization | Decrease | Throughout low glucose treatment | ≥50% | 7 (17) | 54 (18) |
| GI | Depolarization | Increase | 19 (45) | 81 (28) | ||
| AdGI | Depolarization | Increase | Reversed prior to solution change | 9 (21) | 98 (33) | |
AdGI: adapting GI, GE: glucose-excited, GI: nonadapting GI, IR: input resistance, NA: not applicable, NGS: non-glucose sensing, Vm: membrane potential.
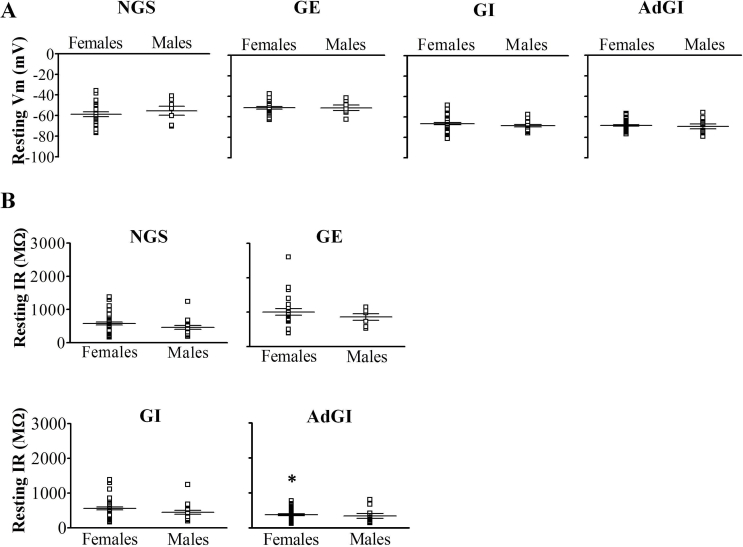
In total, 83% (n = 42) and 80% (n = 293) of VL-VMN neurons were glucose sensitive in males and females, respectively. Importantly, the relative percentage of glucose sensitive subtypes (AdGI, nonadaptive GI, GE, and NGS) within the VL-VMN was sexually dimorphic (Table 1). In the female VL-VMN, AdGI, and nonadapting GI neurons were encountered in roughly equally percentages whereas in the male AdGI neurons were encountered approximately half as frequently as nonadapting GI neurons. In contrast, the percentage of GE and NGS neurons VL-VMN was similar between the sexes. No significant sex differences in the resting Vm or IR of VL-VMN NGS, GE, GI, or AdGI neurons were observed (Supplemental Figure 2).
3.2. VL-VMN nonadapting GI neurons are inherently sexually dimorphic
Male and female nonadapting GI neurons depolarized to a similar degree in response to a glucose decrease from 2.5 to 0.1 mM (male: 10.2 ± 1.5%, n = 18; female 10.6 ± 1.0%, n = 48; p > 0.05). However, the increase in IR in response to this glucose decrease was 2-fold greater in male versus female nonadapting GI neurons (male: 96.5 ± 17.8%, n = 18; female: 52.9 ± 5.5%, n = 48; p < 0.05 Figure 2A–C). Although the degree of depolarization was similar in both sexes, a greater change in IR in male nonadapting GI neurons suggests increased excitability of these neurons in low glucose. There was no sex difference observed in response to a more minimal glucose decrease from 2.5 mM to 0.5 mM glucose (data not shown; p > 0.05).
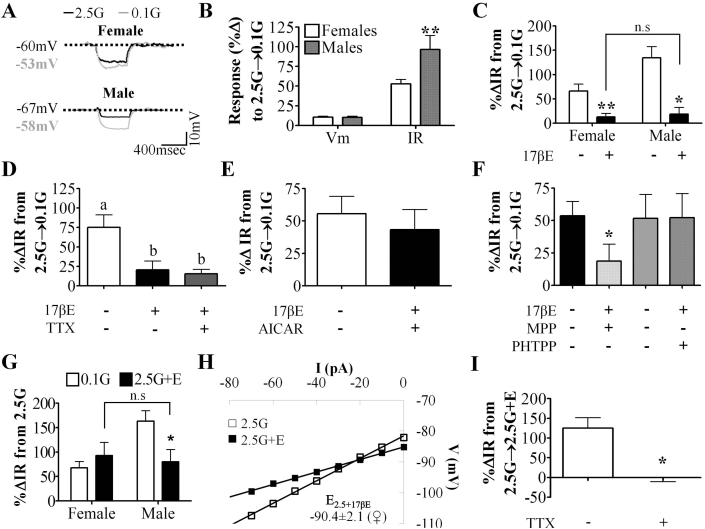
Figure 2.
VL-VMN nonadapting GI neurons are inherently sexually dimorphic and 17βE sensitive. (A) Representative voltage responses to a hyperpolarizing pulse for nonadapting GI neurons from both sexes. Vm was normalized to 2.5 mM glucose to emphasize changes in IR. (B) Quantification of %ΔVm and %ΔIR in response to 0.1 mM (♀n = 48, ♂n = 18) glucose in nonadapting GI neurons from both sexes. (C) Quantification of %ΔIR in response to 0.1 mM glucose in the presence and absence of 17βE (100 nM) for nonadapting GI neurons from females (n = 9) and males (n = 6). n.s: not significant via unpaired students t-test. (D) Quantification of %ΔIR in response to 0.1 mM glucose in the presence and absence of TTX (n = 5) and 17βE (n = 5). Columns with different letters are significantly different from each other as determined by repeated measures one-way ANOVA followed by Tukey post-hoc tests. (E, F) Quantification of %ΔIR in response to 0.1 mM glucose in the presence and absence 17βE and AICAR, (E, n = 4), MPP (F, n = 5) or PHTPP (F, n = 6). *p < 0.05 via unpaired students t-test. (G) Quantification of %ΔIR in response to 0.1 mM glucose and 2.5 mM glucose+17βE in nonadapting GI neurons from females (n = 12) and males (n = 5). n.s: not significant via unpaired students t-test; p < 0.05 via paired students t-test. (H) Representative 17βE-sensitive V-I relationship in 2.5 mM glucose in female nonadapting GI neurons (n = 9). The 17βE-sensitive conductance in 2.5 mM glucose reversed near the K+ equilibrium potential (EK+ = −99 mV) for our solutions. (I) Quantification of %ΔIR in response to 2.5G+17βE (n = 4) in the presence and absence of TTX. *p < 0.05 via paired students t-test. 17βE:17β-Estradiol (100 nM), AICAR: AMPK agonist (0.5 mM), G: mM glucose, IR: input resistance, MPP: ERα antagonist (10 μM), PHTPP: ERβ antagonist (1 μM), TTX: tetrodotoxin (voltage-gated Na+ channel blocker; 500 nM), Vm: membrane potential.
3.3. 17βE blunts the response of VL-VMN nonadapting GI neurons to low glucose
17βE blunted the increased IR of female and male nonadapting GI neurons in response to a glucose decrease from 2.5 mM to 0.1 mM (Figure 2C). Moreover, in the presence of 17βE the response of female and male nonadapting GI neurons to decreased glucose was no longer statistically different (Figure 2C; p > 0.05). Because 17βE attenuated the response of nonadapting GI neurons to decreased glucose from 2.5 to 0.1 mM similarly in both sexes, we studied the mechanism by which 17βE modulates glucose sensitivity in females only. The effect of 17βE persisted in the presence of TTX suggesting that it is a postsynaptic effect (Figure 2D). We hypothesized that, like leptin [12], [16], 17βE may blunt the response of nonadapting GI neurons to decreased glucose via AMPK inhibition. Consistent with this hypothesis, the AMPK activator, AICAR (0.5 mM), blocked the effect of 17βE on glucose sensitivity (Figure 2E).
To begin to explore the mechanism by which 17βE impacts glucose sensitivity, we determined which ER is responsible for this observed 17βE regulation. The effect of 17βE on glucose sensitivity persisted in the presence of the ERα antagonist, MPP; however, the ERβ antagonist PHTPP completely blocked this effect of 17βE (Figure 2F). In 2.5 mM glucose, only PHTPP independently caused a slight increase in IR, (MPP: p = 0.17, PHTPP: p = 0.02, AICAR: p = 0.07; Supplemental Figure 1C). Taken together, the above data suggest that 17βE blunts the response of nonadapting GI neurons to a glucose decrease from 2.5 mM to 0.1 mM directly via ERβ mediated AMPK inhibition.
3.4. 17βE excites a subpopulation of VL-VMN nonadapting GI neurons via a presynaptic mechanism
In 2.5 mM glucose, 17βE depolarized and increased input resistance in 46% (12 of 26) and 42% (5 of 12) of nonadapting GI neurons in females and males (Figure 2G). In female nonadapting GI neurons, the addition of 17βE to 2.5 mM glucose and decreasing glucose from 2.5 mM to 0.1 mM activated these neurons to a similar degree (Figure 2G, n = 7). However, in male nonadapting GI neurons, the response to the addition of 17βE to 2.5 mM glucose was only about half of the magnitude of the response to a decrease in glucose from 2.5 mM to 0.1 mM (Figure 2G, n = 5). This apparent sex difference is most likely not due to differing 17βE sensitivity or ER expression, but rather because the response of male nonadapting GI neurons to low glucose in the absence of 17βE is roughly twice that of females.
Because there was no difference in 17βE-induced excitation of nonadapting GI neurons from males and females in 2.5 mM glucose, we evaluated the mechanism underlying this effect in females only. In 2.5 mM glucose, the 17βE-sensitive conductance reversed at −90.4 ± 2.1 mV (Figure 2H, n = 9). This reversal is near the theoretical K+ equilibrium potential in our solutions (EK+ = −99 mV). TTX completely abolished the 17βE-mediated excitation in 2.5 mM glucose (Figure 2I) suggesting 17βE excites this subpopulation of nonadapting GI neurons through a presynaptic mechanism.
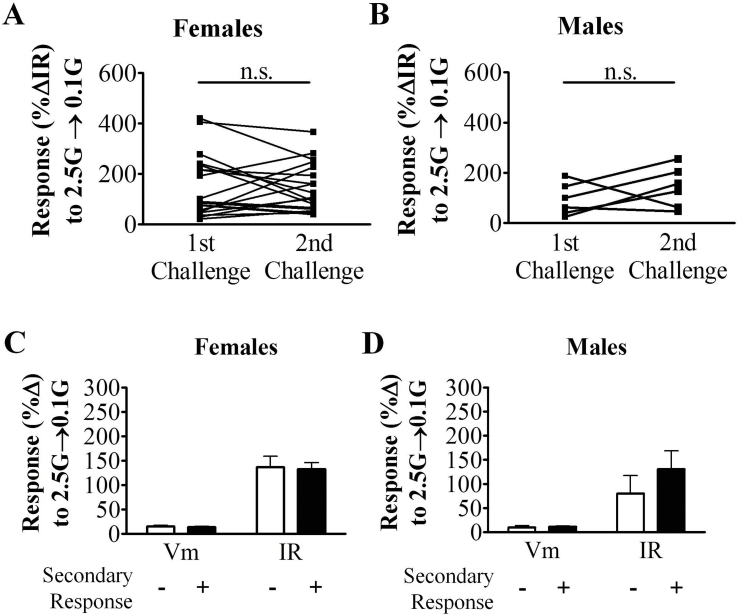
3.5. Identification of VL-VMN adapting GI neurons
Within the VL-VMN, a type of glucose sensitive cell was identified which has not been previously described in the VMN. AdGI neurons were excited when glucose was lowered from 2.5 mM to 0.1 mM, and this response reversed prior to restoration of the original glucose solution (Figure 1D). The magnitude of activation (Figure 3A), duration of activation (∼1 min in both sexes), and the time to maximal response (∼4–5 min post-solution change in both sexes) were not sexually dimorphic. In response to two consecutive glucose challenges (2.5 mM–0.1 mM), both female and male AdGI neurons were activated to the same magnitude at each glucose challenge (♀n = 19, ♂n = 6; Supplemental Figure 3A and B). Female AdGI neurons were more robustly activated in response to a glucose decrease from 2.5 mM to 0.1 mM glucose than female nonadapting GI neurons (Figure 3B). That is, in response to this glucose decrease, female AdGI neurons depolarized by 12.6 ± 0.6% (n = 92) and increased their input resistance by 115.6 ± 10.4% compared to 8.3 ± 0.8% (n = 69) and 53.5 ± 4.1% in female nonadapting GI neurons. In contrast, male nonadapting GI and AdGI neurons responded similarly to a glucose decrease from 2.5 mM to 0.1 mM (Figure 3B).
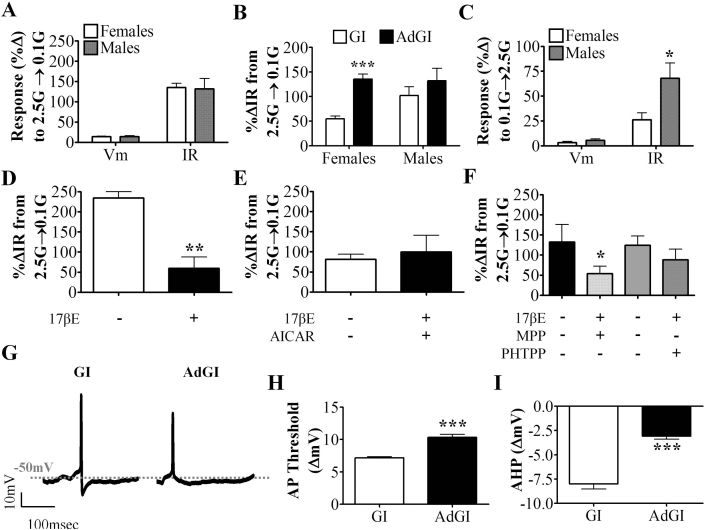
Figure 3.
VL-VMN adapting GI (AdGI) neurons are not inherently sexually dimorphic but are 17βE sensitive. (A) Quantification of %ΔVm and %ΔIR in response to 0.1 mM glucose in both sexes (♀n = 47, ♂n = 9). (B) Comparison of %ΔIR in response to 0.1 mM glucose in nonadapting GI and AdGI neurons from females (n = 45 GI, 47 AdGI) and males (n = 17 GI, 9 AdGI). ***p < 0.001 via unpaired student t-test. (C) Quantification of a secondary glucose response (%ΔVm and %ΔIR) in AdGI neurons from both sexes (♀n = 18, ♂n = 6). *p < 0.05 via unpaired student t-test. (D–F) Quantification of %ΔIR in response to 0.1 mM glucose in the presence and absence of 17βE (D, n = 5), and AICAR (E, n = 5), MPP (F, n = 6) or PHTPP (F, n = 5). 17βE: 17β-estradiol, AICAR: AMPK activator (0.5 mM), *p < 0.05 via paired student t-test; **p < 0.01, via paired student t-test. G: mM glucose, IR: input resistance, MPP: ERα antagonist (10 μM), PHTPP: ERβ antagonist (1 μM), Vm: membrane potential. (G) Representative action potentials generated in female nonadapting GI and AdGI neurons. Dashed grey line represents Vm. (H, I) Quantification of action potential threshold (H; n = 42 GI, 74 AdGI events from n = 3 neurons each) and afterhyperpolarization magnitude (I; n = 38 GI, 43 AdGI events from n = 3 neurons each) in 0.1 mM glucose for nonadapting GI and AdGI neurons. ***p < 0.001 via unpaired student t-test. AHP: afterhyperpolarization, AP: action potential, IR: input resistance, Vm: membrane potential.
Interestingly, approximately 60% of male (n = 6 of 10) and 35% of female (n = 18 of 51) AdGI neurons showed a smaller amplitude transient depolarization in response to a subsequent glucose challenge from 0.1 mM to 2.5 mM (Figure 1D inset). This transient depolarization was sexually dimorphic. That is, in response to this glucose increase, male AdGI neurons depolarized and increased their input resistance by 5.6 ± 1.4% and 67.9 ± 15.5% versus 4.1 ± 1.0% and 27.6 ± 3.3% in female AdGIs (Figure 3C; p < 0.05). No sex differences were observed in the time to peak or duration of this secondary glucose response in AdGI neurons and these parameters were similar to those observed in response to 0.1 mM glucose. Furthermore, no significant differences in the response to a glucose decrease were observed between AdGI exhibiting or not exhibiting this secondary response in either sex (Supplemental Figure 3C and D). These data suggest that a subpopulation of AdGI neurons may potentially respond to an absolute change in glucose instead of a unidirectional change. However, we designate them as GI neurons because their activation in low glucose is far more pronounced than their activation when glucose is subsequently raised (Figure 1D).
3.6. 17βE blunts the response of VL-VMN AdGI neurons to low glucose
Because AdGI neurons were not sexually dimorphic with respect to activation in low glucose (Figure 3A), we only examined the effects of 17βE on the glucose sensitivity of female AdGI neurons. In contrast to nonadapting GI neurons, 17βE (100 nM) had no effect on AdGI neurons in 2.5 mM glucose (n = 5, data not shown). However, when glucose was lowered from 2.5 mM to 0.1 mM in the presence of 17βE, the IR response to low glucose of AdGI neurons was attenuated (Figure 3D). Furthermore, like nonadapting GI neurons, AICAR blocked this effect of 17βE on AdGI neurons (Figure 3E). This suggests that 17βE may attenuate the response of AdGI neurons to decreased glucose by blunting AMPK activation.
The ER responsible for 17βE's effect was evaluated as in nonadapting GI neurons. 17βE blunted the increased IR of AdGI neurons in 0.1 mM glucose in the presence of the ERα antagonist, MPP, but not the ERβ antagonist, PHTPP (Figure 3F). Neither the ER antagonist nor AICAR affected the resting IR of AdGI neurons (MPP: p = 0.06, PHTPP: p = 0.52, AICAR: p = 0.16; Supplemental Figure 1D). Thus, as in nonadapting GI neurons, 17βE's effect on glucose sensing appears to be mediated by ERβ-AMPK signaling.
3.7. Biophysical characterization of VL-VMN adapting GI (AdGI) neurons
Resting membrane potential was similar in nonadapting GI and AdGI neurons in both sexes; however, resting input resistance was lower in female AdGI versus nonadapting GI neurons (nonadapting GI neurons: 566 ± 45 MΩ, AdGI: 373 ± 25 MΩ; p < 0.05, Supplemental Figure 2). As we have observed previously for VMN nonadapting GI neurons, only a small proportion of VL-VMN nonadapting and AdGI neurons actually initiate action potentials in low glucose [11]. This is most likely due to the in vitro experimental conditions rather than a physiologic characteristic of these neurons. In the current study, approximately 20% of male (2 of 9) and female (19 of 92) AdGI neurons initiated action potentials in response to low glucose. Similarly, 11% of (2 of 17) and 20% (14 of 69) of male and female nonadapting GI neurons, respectively, initiated action potentials in response to low glucose. The action potential frequency (APF) of female nonadapting GI and AdGI neurons in 0.1 mM glucose was similar (31.7 ± 7.5 Hz; n = 15 and 30.9 ± 7.3 Hz; n = 13, respectively); however, the shape of the action potentials generated by these neurons differed (Figure 3G). Moreover, AdGI neurons required a 10.3 ± 0.5 mV (n = 74) depolarization for action potential initiation whereas nonadapting GI neurons only required a 7.2 ± 0.2 mV (n = 42) depolarization (Figure 3H). AdGI neurons also had a less pronounced after-hyperpolarization (AHP) than nonadapting GI neurons (Figure 3I), but AHP duration did not differ (∼4–5 ms; data not shown). Similar differences in the action potential shape of male nonadapting GI and AdGI neurons were observed; however, this was not quantified in males due to the paucity of AdGI neurons exhibiting action potentials (n = 2; data not shown).
3.8. VL-VMN NGS and GE neurons are not sexually dimorphic
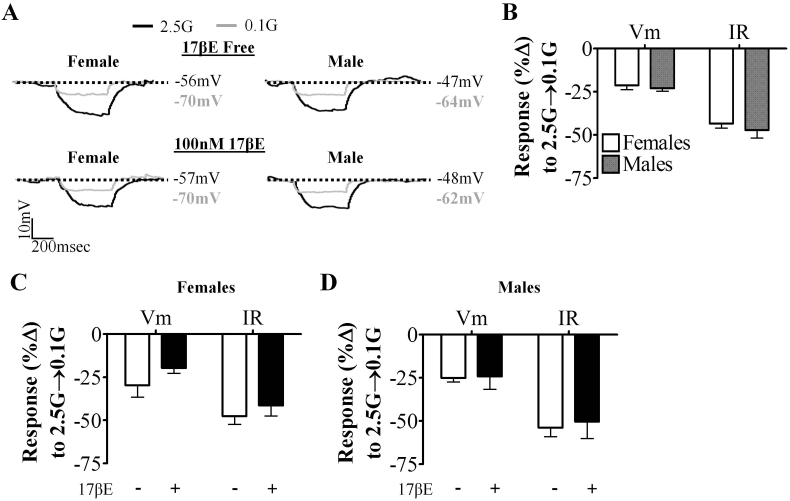
A small population of NGS and GE neurons met our criteria for 17βE sensitivity as defined in the methods and Supplemental Figure 1A. In 2.5 mM glucose, 17βE excited 11% (1 of 9) of female and 0% (0 of 3) of male NGS neurons. Similarly, a subpopulation of GE neurons, 18% (2 of 11) and 20% (1 of 5) in female and males, respectively, were also excited by 17βE in 2.5 mM glucose.
Female and male GE neurons responded similarly to a glucose decrease from 2.5 mM to 0.1 mM glucose (Figure 4A and B). As reported previously [11], the glucose-sensitive conductance in GE neurons reversed near the theoretical K+ equilibrium potential in our solutions (EK+ = −99 mV; ♀: 92.2 ± 2.5 mV (n = 7), ♂: 90.7 ± 3.9 mV (n = 5) and was blocked by tolbutamide (n = 3); data not shown). 17βE had no effect on the glucose sensitivity of either male or female GE neurons (Figure 4C,D). Given that NGS and GE neurons were not sexually dimorphic and rarely 17βE-sensitive in 2.5 mM glucose and that 17βE had no effect on the glucose sensitivity of VL-VMN GE neurons, we did not investigate these neurons further.
Figure 4.
VL-VMN GE neurons are neither sexually dimorphic nor 17βE sensitive. (A) Representative voltage responses to a hyperpolarizing pulse for GE neurons from both sexes. Vm was normalized to 2.5 mM glucose to emphasize changes in IR. (B) Quantification of %ΔVm and %ΔIR in response to 0.1 mM glucose in VL-VMN GE neurons from both sexes (♀n = 25, ♂n = 7). (C, D) Quantification of %ΔVm and %ΔIR in response to 0.1 mM glucose in the presence and absence of 17βE in females (C, n = 4) and males (D, n = 3). 17βE: 17β-estradiol, G: mM glucose, IR: input resistance, Vm: membrane potential.
3.9. AMPK activation and K+ channel closure mediates glucose sensing in VL-VMN nonadapting GI and AdGI neurons
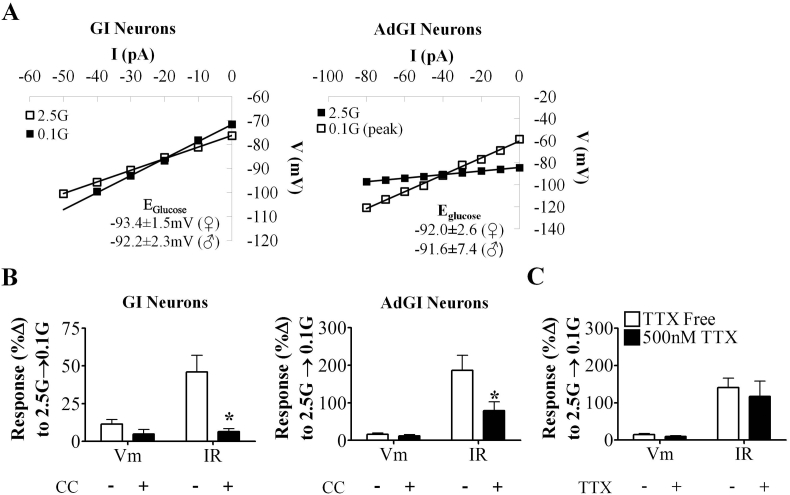
We hypothesized that VL-VMN GI neurons sense glucose deficit similar to nonadapting GI neurons in the dorsomedial VMN (DM-VMN) i.e., via activation of AMPK and closure of a Cl− channel [12]. However, the glucose-sensitive conductance in VL-VMN nonadapting GI neurons reversed at −93.4 ± 1.5 mV (n = 13) and −92.2 ± 2.3 mV (n = 7) in females and males, respectively (Figure 5A). A similar reversal potential was observed in AdGI neurons (♀Eglucose = −92.0 ± 2.6 mV; n = 6 and ♂Eglucose = −91.6 ± 7.3 mV; n = 3). This reversal potential is near the theoretical K+ equilibrium potential in our solutions (EK+ = −99 mV). In females, the AMPK inhibitor, CC, blocked the effect of 0.1 mM glucose in both types of GI neurons (Figure 5B). Activation of female AdGI neurons in 0.1 mM glucose persisted in the presence of TTX (Figure 5C), suggesting AdGI neurons directly sense glucose fluctuations. In 2.5 mM glucose, CC caused a slight increase in IR in both GI neuron subtypes (GI: p = 0.01, AdGI: p = 0.04; Supplemental Figure 1C and D). Together, these data suggest that like DM-VMN nonadapting GI neurons, both VL-VMN nonadapting GI and AdGI neurons sense glucose deficit directly via changes in AMPK activity However, AMPK signaling in GI neurons from the VL-VMN is coupled to K+ rather than Cl− channels.
Figure 5.
VL-VMN nonadapting GI neurons and AdGI utilize an AMPK-dependent glucose sensing mechanism. (A) Representative glucose-sensitive V-I relationship in nonadapting GI neurons (left; ♀n = 13, ♂n = 7) and AdGI neurons (right; ♀n = 6, ♂n = 3). The glucose-sensitive conductance reversed near the K+ (EK+ = −99 mV) equilibrium potential for our solutions. (B) Quantification of %ΔVm and %ΔIR in response to 0.1 mM glucose in the presence and absence of CC in female nonadapting GI neurons (left; n = 6) and female AdGI neurons (right; n = 6). (C) Quantification of %ΔVm and %ΔIR in response to 0.1 mM glucose in of female AdGI neurons in the presence and absence of TTX (n = 7) *p < 0.05 via paired student t-test. AMPK: AMP-activated kinase, CC: Compound C (AMPK antagonist; 10 μM), G: mM glucose, IR: input resistance, TTX: tetrodotoxin (voltage-gated Na+ channel antagonist; 500 nM), Vm: membrane potential.
3.10. 17βE modulates VMH AMPK phosphorylation via a membrane-bound ER
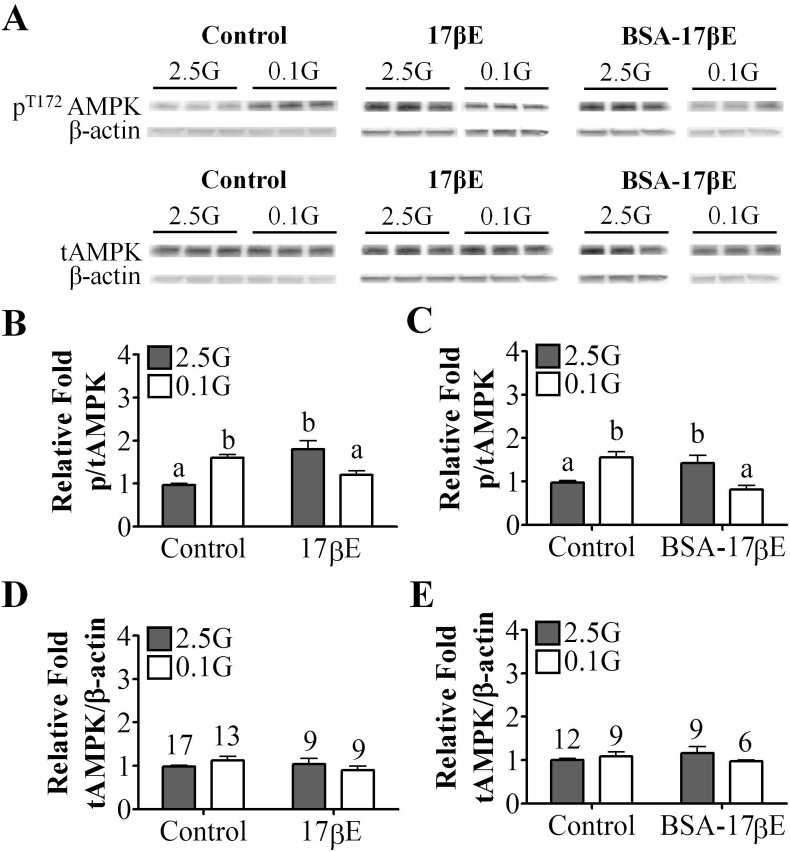
Based on the electrophysiological evidence above, we hypothesized that 17βE blunts the activation of nonadapting GI and AdGI neurons in low glucose by attenuating AMPK phosphorylation. In whole VMH tissue, a decrease in glucose from 2.5 mM to 0.1 mM glucose increased AMPK Thr172 phosphorylation consistent with activation of this cellular energy sensor (Figure 6A–C). Unexpectedly, acute application (30 min) of 17βE or its membrane impermeable analog, BSA-17βE, in 2.5 mM glucose also increased AMPK Thr172 phosphorylation (Figure 6B and C). However, when glucose was lowered from 2.5 mM to 0.1 mM glucose in the presence of 17βE or BSA-17βE the normal increase in AMPK Thr172 phosphorylation was blunted (Figure 6Band C). Total AMPK expression was unaffected by 17βE or BSA-17βE application (Figure 6D and E).
Figure 6.
In females, 17β-estradiol modulates whole VMH phospho (p)-AMPK levels via a membrane-bound ER. (A) Representative western blot images for pT172-AMPK, tAMPK, and β-actin. (B–E) Quantification of relative fold expression of whole VMH pAMPK (B, C) and total (t)-AMPK (D, E) in the presence and absence of 17βE or BSA-17βE. Numbers above columns indicate the n of each group. Columns with different letters are significantly different from each other as determined by two-way ANOVA followed by Bonferroni post-hoc tests. 17βE: 17β-estradiol (100 nM), AMPK:AMP-activated kinase, BSA-17βE: bovine serum albumin-conjugated 17βE (100 nM), ER: estrogen receptor, G: mM glucose, VMH: ventromedial hypothalamus.
4. Discussion
This study is the first to demonstrate sexual dimorphism and the impact of 17βE modulation in hypothalamic glucose sensing. We show that in the absence of 17βE, VL-VMN nonadapting GI neurons from females increase their IR in low glucose to a lesser degree than those from age and weight matched males. In addition, we found that as for the lateral hypothalamic orexin neurons [33], the VL-VMN possesses a subpopulation of GI neurons whose response to low glucose is transient despite continued exposure (AdGI neurons). 17βE modulated the activity and/or the glucose sensitivity of both VL-VMN nonadapting GI and AdGI neurons. In contrast, GE and NGS neurons were neither sexually dimorphic nor 17βE sensitive to any significant degree. Our data suggest that the glucose sensitivity of VL-VMN nonadapting GI, but not other glucose or NGS, neurons is inherently sexually dimorphic (due to organizational effects). Moreover, for both nonadapting GI and AdGI neurons, hormonal effects (the presence of 17βE) may further contribute to sex differences in the response of these neurons to low glucose. Interestingly, over 80% of VL-VMN neurons are glucose sensitive, making the VL-VMN the most concentrated brain locus for glucose sensitive cells identified to date. Thus, inherent sex differences and 17βE modulation of VL-VMN GI and AdGI neurons may play a role in the sexual dimorphism observed in glucose homeostasis and hypoglycemia counterregulation [9], [34].
Our finding that nonadapting GI neurons from females show a smaller increase in IR in response to low glucose compared to those from males suggests altered responsiveness to presynaptic input. IR is a measure of how much current is needed to change Vm by a given amount. Thus, in low glucose, it would take a larger presynaptic current to change the Vm of nonadapting GI neurons from females to the same degree as for males. This is consistent with the sex differences in hypoglycemia detection and counterregulation observed in both humans and rodents [9], [34]. Specifically, sympathetic drive [6], glucagon [7] and epinephrine secretion [8], [9], and hepatic glucose output [10] are significantly lower in females than in males. Central regulation of hypoglycemia counterregulation is a complex interplay of input from multiple brain regions [35]. However, the VMH is a critical component of this system. Local VMH glucopenia [4] or glucose infusion [5] initiates or attenuates the peripheral release of counterregulatory hormones, respectively. Our lab has previously shown that VMN nonadapting GI neurons, in particular, play a role in hypoglycemia counterregulation [12], [13], [14]. Here, we demonstrate that nonadapting VL-VMN GI neurons from males respond more robustly to a large glucose decrease from 2.5 mM to 0.1 mM glucose than those from females. This suggests that there are inherent sex differences in the response to low glucose in nonadapting VL-VMN GI neurons. On the other hand, the response of nonadapting VL-VMN GI neurons to a smaller glucose decrease (2.5 mM–0.5 mM) was similar in both sexes. Interestingly, in the presence of 17βE, the response of nonadapting GI neurons to low glucose was nearly abolished in both males and females. These data suggest that, in general, the mechanisms underlying hypoglycemia detection may be similar in male and females, but maximal responsiveness of this neurocircuitry may be less in females due to inherent sex differences. The presence of estrogens in females would further attenuate the response of these neurons to low glucose. Thus, during a hypoglycemic event, higher 17βE levels in females in addition to the underlying sex difference may prevent nonadapting GI neurons from sensing decreased glucose. This could blunt the sympathoadrenal and hormonal counterregulatory response causing blood glucose levels to drop lower in females than in males. Similarly, natural fluctuations in 17βE levels during the female reproductive cycle may also alter hypoglycemia counterregulation. Caution must be taken when extrapolating the results of this ex-vivo study to an in-vivo setting due to the use of a supraphysiological 17βE concentration in this study [28].
In the lateral hypothalamus, ∼70% of orexin GI neurons transiently hyperpolarized when glucose increased above 2.5 mM [33]. We now describe a heretofore uncharacterized subpopulation of VL-VMN GI neurons (AdGI neurons) that transiently depolarize when glucose decreases below 2.5 mM. Adaptation in orexin GI neurons is thought to be analogous to rapid adaptation in certain peripheral sensory receptors (e.g., Pacinian corpuscles [36]). These receptors enable the nervous system to be more attentive to a change in stimulus rather than its constant presence. Consistent with this hypothesis, we observed a small depolarization in some VL-VMN AdGI neurons when glucose was subsequently raised to 2.5 mM glucose. Thus, VL-VMN AdGI neurons may serve a similar function and preserve brain glucose sensitivity during prolonged periods of glucose deficit.
The glucose sensing mechanism in VL-VMN nonadapting GI and AdGI neurons overlaps to some extent with that of other VMN GI neurons. That is, we have previously shown that low glucose excites VMN GI neurons through AMPK activation and closure of a Cl− channel [11], [12], [37]. Consistent with these findings, we found that the AMPK inhibitor, CC, blocks glucose sensing in both VL-VMN nonadapting GI and AdGI neurons. However, in these VL-VMN GI neurons AMPK activation closes a K+ rather than a Cl− channel. These data suggest that while AMPK appears to mediate glucose sensing in VMN GI neurons independent of subtype, AMPK signaling is coupled to different ion channels among distinct subtypes. One caveat to this conclusion is the potential for known off-target effects of CC [38], [39]. However, despite this drawback, CC is a competitive inhibitor of ATP regulation of AMPK [40]. Thus, it interacts with the metabolic pathways which would be affected by changing glucose levels. In this regard, the fact that CC blocked the effects of low glucose, although not definitive, is consistent with AMPK mediated glucose sensing in AdGI neurons.
Glucose-sensitive K+ channels have been previously described in the hypothalamus [41], [42]; however, the specific K+ channel involved is as yet unknown [43], [44], [45]. The female VL-VMN contains fewer nonadapting GI but more AdGI neurons than the male VL-VMN. Furthermore, the secondary glucose response of AdGI neurons to a glucose increase was smaller in females than in males. It is possible that, in females versus males, the net output of the hypoglycemia sensitive neurocircuit is dampened in response to prolonged insulin-induced hypoglycemia since the overall activation of nonadapting GI neurons is blunted in females and fewer GI neurons overall maintain activation during the entire hypoglycemic episode. Moreover, while glucose sensing in AdGI neurons was not itself sexually dimorphic, 17βE blunted their response to low glucose. Thus, the presence of estrogens in the female would further dampen the nonadapting GI and AdGI hypoglycemia sensitive neurocircuit and contribute to the reduced hypoglycemia counterregulation observed in females compared to males.
Interestingly, the cumulative concentration of glucose sensing neurons (GE, nonadapting GI and AdGI) in the VL-VMN is greater than in any other brain region so far examined. That is, over 80% of VL-VMN neurons are glucose sensing neurons. As described previously, 20% are GE neurons [15], with GI and AdGI making up the remainder. This is in comparison to our previous finding that less than 20% of the neurons in the DM-VMN respond to glucose changes [11]. The dense concentration of glucose sensing neurons in the VL-VMN suggests that these neurons may play a greater role in linking glucose availability with whole body metabolism and/or reproductive function in this brain area than previously considered [45]. The volume of the VMN is larger in males, but neuron density is similar in both sexes [46]. The high concentration of both estrogen receptors and GI neurons in the VL-VMN suggests that VL-VMN GI neurons, which are more prominent in the male VL-VMN than the female VL-VMN may mediate, in part, the sexually dimorphic effects of this brain region on glucose homeostasis. That is, the overall activation of the male VL-VMN during hypoglycemia may be greater than that in the female. This would be again consistent with blunted hypoglycemia counterregulation in the female compared to the male.
As discussed above, the ability of 17βE to blunt the activation of nonadapting GI and AdGI neurons in low glucose is blocked by an AMPK activator. This is consistent with our finding that 17βE and its membrane impermeable analog also blocked the low glucose-induced AMPK Thr172 phosphorylation in VMH tissues sections. While the expression of ERs on VL-VMN glucose sensing neurons has yet to be shown, these data suggest that 17βE inhibits the glucose sensing signaling pathway (AMPK) via a membrane bound (vs nuclear) ER. Based on our pharmacological data this membrane bound receptor appears to be ERβ instead of ERα. ERβ null animals display no altered metabolic phenotype and remain fertile [47], [48]. However, chronic intermittent insulin-induced hypoglycemia down regulates ARC ERβ expression while upregulating ARC ERα expression [49]. If this occurred in VL-VMN nonadaptive GI or AdGI neurons, it could blunt 17βE's effect on glucose sensitivity and protect counterregulation. This would be consistent with the observation that females are protected to some degree against the deleterious effects of antecedent hypoglycemia on counterregulation in comparison to males [50]. Alternatively, ERβ-mediated phosphoinositide 3-kinase (PI3K) activation may mediate 17βE's effect on VL-VMN glucose sensing [51].
In contrast to its inhibitory effect in low glucose, 17βE depolarizes approximately half of the nonadapting GI neurons in 2.5 mM glucose while having no effect on AdGI neurons in this glucose concentration. This apparent paradoxical depolarizing effect of 17βE in nonadapting GI neurons was found to be due to a presynaptic site of action since it was blocked by the voltage-gated sodium channel blocker TTX, which blocks presynaptic action potentials. The effect of 17βE in 2.5 mM glucose was due to a decrease in K+ conductance, suggesting that the neurotransmitter released from the upstream 17βE-sensitive neuron is coupled to a K+ channel on nonadapting GI neurons. γ-Aminobutyric acid (GABA) and the K+ channel linked GABAB receptor are plausible candidates. 17βE downregulates hypothalamic GABAB receptors and decreases the potency of the GABAB agonist baclofen in VMH neurons [52], [53]. Reduced GABAergic input onto VL-VMN nonadapting GI neurons could close K+ channels, leading to excitation of these neurons. However, this remains to be tested, and there are admittedly other candidates, which could explain these results. It is possible that the neurotransmitter released from the 17βE-sensitive presynaptic neuron, be it GABA or otherwise, increases AMPK activity leading to neuronal activation. This would be consistent with our finding that 17βE increased AMPK Thr172 phosphorylation in 2.5 mM glucose. However, these data must be interpreted cautiously since 17βE-induced modulation of AMPK phosphorylation in other VMH AMPK expressing neurons [54], [55] or astrocytes [56] must be taken into account in VMH tissue sections. Moreover, since the brain slices from which we record have been isolated from their normal interconnections with the rest of the brain, it is difficult to extend presynaptic effects in brain slices to the physiological situation in vivo.
In conclusion, we have shown a sexual dimorphism in nonadapting VL-VMN GI neurons, which is apparently due to organizational effects since differences are present in the absence of estrogens. Moreover, 17βE affects the glucose sensing machinery (AMPK) in both nonadapting GI and AdGI neurons of both sexes. Together, our data suggest that sex differences observed in VL-VMN nonadapting GI neurons and 17βE effects on the glucose sensitivity of both nonadaptive GI and AdGI neurons may underlie the observed sex differences in hypoglycemia detection and counterregulation.
Acknowledgements
This study was supported by National Institutes of Health DK081538 (VHR) and NRSA 5R31DK093331 (AMS).
Footnotes
Supplemental Figures related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2016.08.002.
Contributor Information
Ammy M. Santiago, Email: santiaa1@njms.rutgers.edu.
Deborah J. Clegg, Email: Deborah.Clegg@cshs.org.
Vanessa H. Routh, Email: routhvh@njms.rutgers.edu.
Conflict of interest
None.
Appendix A. Supplemental Figures
The following are the Supplemental Figures related to this article:
Supplemental Figure 1.
Solution change, vehicle-only, and pharmacological drug-only controls. (A) Pooled data scatter plot of %ΔIR of VL-VMN neurons during control solution change from both females (n = 13) and males (n = 6). (B) Quantification of %ΔIR of VL-VMN neurons in response to vehicle controls pooled from both sexes. Numbers above columns indicate the n for each group. (C, D) Quantification of %ΔIR in response to MPP, PHTPP, AICAR and CC in 2.5 mM glucose for nonadapting GI (C) and AdGI neurons (D) from females only. Numbers above columns indicate the n number for each group. Shaded boxes represent 2xSTD of control solution change experiments (see Supplemental Figure 1A). Treatments exhibiting %ΔIR responses within this shaded area were deemed as “no effect.” *p < 0.05 via one-sample student t-test with a theoretical mean = 0. AICAR: AMPK agonist (0.5 mM), CC: AMPK antagonist (10 μM), G: mM glucose, ER: estrogen receptor, IR: input resistance, MPP: ERα antagonist (10 μM), PHTPP: ERβ antagonist (1 μM), Res: 2.5G from general reservoir, Syr: 2.5G from treatment syringe, Vm: membrane potential.
Supplemental Figure 2.
Basal resting Vm and IR in VL-VMN glucose sensitive cells from both sexes. (A, B) Scatter plot of resting Vm (A) and IR (B) in 2.5 mM glucose for VL-VMN NGS (♀n = 26, ♂n = 7), GE(♀n = 25, ♂n = 7), nonadapting GI (♀n = 47, ♂n = 18) and AdGI (♀n = 47, ♂n = 10) neurons from both sexes. *p < 0.05 versus female nonadapting GI neurons via unpaired student t-test; all other comparisons were not statistically significant.
Supplemental Figure 3.
Characterization of consecutive glucose challenges and the secondary glucose response in VL-VMN AdGI neurons. (A, B) Scatter plot of %ΔIR in AdGI neurons from females (A; n = 19) and males (B; n = 6). n.s: not significant via paired students t-test (C, D) Quantification of %ΔVm and %ΔIR in response to 0.1 mM glucose in AdGI exhibiting or not exhibiting a secondary glucose response in females (C, n = 18 of 51) and males (D, n = 6 of 10). G: mM glucose, IR: input resistance, Vm: membrane potential.
References
- 1.Kelly M.J., Qiu J. Estrogen signaling in hypothalamic circuits controling reproduction. Brain Research. 2010 doi: 10.1016/j.brainres.2010.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank A., Brown L.M., Clegg D.J. The role of hypothalamic estrogen receptors in metabolic regulation. Frontiers in Neuroendocrinology. 2014;35:550–557. doi: 10.1016/j.yfrne.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu Y., Nedungadi Thekkethil P., Zhu L., Sobhani N., Irani Boman G., Davis Kathryn E. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metabolism. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borg W.P., Sherwin R.S., During M.J., Borg M.A., Shulman G.I. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes. 1995;44:180–184. doi: 10.2337/diab.44.2.180. [DOI] [PubMed] [Google Scholar]
- 5.Borg M.A., Sherwin R.S., Borg W.P., Tamborlane W.V., Shulman G.I. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. The Journal of Clinical Investigation. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis S.N., Shavers C., Costa F. 2000. Differential gender responses to hypoglycemia are due to alterations in CNS drive and not glycemic thresholds. [DOI] [PubMed] [Google Scholar]
- 7.Cox D.J., Gonder-Frederick L.A., Julian D.M., Clarke W.L. 1996. Sex differences in plasma glucose thresholds for counterregulatory hormone release and hypoglycemia symptom perception; pp. 269–270. [DOI] [PubMed] [Google Scholar]
- 8.Drake K., Gateva E., Deutsch J., Cohen W.R. Sex differences in the adrenal catecholamine response to hypoglycemia in rats. Metabolism. 1998;47:121–124. doi: 10.1016/s0026-0495(98)90205-0. [DOI] [PubMed] [Google Scholar]
- 9.Amiel S.A., Maran A., Powrie J.K., Umpleby A.M., Macdonald I.A. Gender differences in counterregulation to hypoglycaemia. Diabetologia. 1993;36:460–464. doi: 10.1007/BF00402284. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson C., Yassin K., Wahlström E., Cheung L., Lindberg J., Brismar K. Sex-different hepaticglycogen content and glucose output in rats. BMC Biochemistry. 2010;11:38. doi: 10.1186/1471-2091-11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Z., Levin B.E., McArdle J.J., Bakhos N., Routh V.H. Convergence of pre- and postsynaptic influcences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2001;50:2673–2681. doi: 10.2337/diabetes.50.12.2673. [DOI] [PubMed] [Google Scholar]
- 12.Murphy B.A., Fakira K.A., Song Z., Beuve A., Routh V.H. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. American Journal of Physiology. Cell physiology. 2009;297:C750–C758. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song Z., Routh V.H. Recurrent hypoglycemia reduces the glucose sensitivity of glucose-inhibited neurons in the ventromedial hypothalamus nucleus. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R1283–R1287. doi: 10.1152/ajpregu.00148.2006. [DOI] [PubMed] [Google Scholar]
- 14.Fioramonti X., Marsollier N., Song Z., Fakira K.A., Patel R.M., Brown S. Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes. 2010;59:519–528. doi: 10.2337/db09-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotero V.E., Routh V.H. Insulin blunts the response of glucose-excited neurons in the ventrolateral-ventromedial hypothalamic nucleus to decreased glucose. American Journal of Physiology. Endocrinology and Metabolism. 2009;296:E1101–E1109. doi: 10.1152/ajpendo.90932.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Canabal D.D., Song Z., Potian J.G., Beuve A., McArdle J.J., Routh V.H. Glucose, insulin and leptin signaling pathways modulate nitric oxide synthesis in glucose-inhibited neurons in the ventromedial hypothalamus. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;292:R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 17.Masuyama H., Hiramatsu Y. Potential role of estradiol and progesterone in insulin resistance through constitutive androstane receptor. Journal of Molecular Endocrinology. 2011;47:229–239. doi: 10.1530/JME-11-0046. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani-Kaneko R. Mechanisms underlying estrogen-induced sexual differentiation in the hypothalamus. Histology & Histopathology. 2006;21:317–324. doi: 10.14670/HH-21.317. [DOI] [PubMed] [Google Scholar]
- 19.Caligioni C.S. Assessing reproductive status/stages in mice. Current Protocols in Neuroscience. 2009;Appendix 4 doi: 10.1002/0471142301.nsa04is48. Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R., Liu X., Hentges S.T., Dunn-Meynell A.A., Levin B.E., Wang W. The regulation of glucose-excited neurons in the hypothalamic arcuate nucleus by glucose and feeding-relevant peptides. Diabetes. 2004;53:1959–1965. doi: 10.2337/diabetes.53.8.1959. [DOI] [PubMed] [Google Scholar]
- 21.Paxinos G., Franklin K.B.J. 2nd ed. Academic Press; San Diego: 2004. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 22.Fioramonti X., Contie S., Song Z., Routh V.H., Lorsignol A., Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 23.Murphy B.A., Fioramonti X., Jochnowitz N., Fakira K., Gagen K., Contie S. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. American Journal of Physiology. Cell physiology. 2009;296:C746–C756. doi: 10.1152/ajpcell.00641.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheng Z., Santiago A.M., Thomas M.P., Routh V.H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Molecular and Cellular Neuroscience. 2014;62:30–41. doi: 10.1016/j.mcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song Z., Routh V.H. Differential effects of glucose and lactate on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes. 2005;54:15–22. doi: 10.2337/diabetes.54.1.15. [DOI] [PubMed] [Google Scholar]
- 26.Silver I.A., Erecinska M. Extracellular glucose concentration in mammalian brain: continuous monitoring of changes during increased neuronal activity and upon limitation in oxygen supply in normo-, hypo-, and hyperglycemic animals. Journal of Neuroscience. 1994;14:5068–5076. doi: 10.1523/JNEUROSCI.14-08-05068.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vries M.G., Arseneau L.M., Lawson M.E., Beverly J.L. Extracellular glucose in rat ventromedial hypothalamus during acute and recurrent hypoglycemia. Diabetes. 2003;52:2767–2773. doi: 10.2337/diabetes.52.11.2767. [DOI] [PubMed] [Google Scholar]
- 28.Kato A., Hojo Y., Higo S., Komatsuzaki Y., Murakami G., Yoshino H. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Frontiers in Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhong Y.Q., Li K.C., Zhang X. Potentiation of excitatory transmission in substantia gelatinosa neurons of rat spinal cord by inhibition of estrogen receptor alpha. Molecular Pain. 2010;6:92. doi: 10.1186/1744-8069-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre C., Jayaraman A., Pike C., Baudry M. Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-beta. Journal of Neurochemistry. 2010;115:1277–1287. doi: 10.1111/j.1471-4159.2010.07038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Temple J.L., Wray S. Bovine serum albumin-estrogen compounds differentially alter gonadotropin-releasing hormone-1 neuronal activity. Endocrinology. 2005;146:558–563. doi: 10.1210/en.2004-1117. [DOI] [PubMed] [Google Scholar]
- 32.Cotero V.E., Zhang B.B., Routh V.H. The response of glucose-excited neurones in the ventromedial hypothalamus to decreased glucose is enhanced in a murine model of type 2 diabetes mellitus. Journal of Neuroendocrinology. 2010;22:65–74. doi: 10.1111/j.1365-2826.2009.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams R.H., Alexopoulos H., Jensen L.T., Fugger L., Burdakov D. Adaptive sugar sensors in hypothalamic feeding circuits. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11975–11980. doi: 10.1073/pnas.0802687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamond M.P., Jones T., Caprio S., Hallarman L., Diamond M.C., Addabbo M. Gender influences counterregulatory hormone responses to hypoglycemia. Metabolism. 1993;42:1568–1572. doi: 10.1016/0026-0495(93)90152-e. [DOI] [PubMed] [Google Scholar]
- 35.Donovan C.M., Watts A.G. Peripheral and central glucose sensing in hypoglycemic detection. Physiology (Bethesda) 2014;29:314–324. doi: 10.1152/physiol.00069.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmerman A., Bai L., Ginty D.D. The gentle touch receptors of mammalian skin. Science (New York, N.Y.) 2014;346:950–954. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Routh V.H., Donovan C.M., Ritter S. 2. Hypoglycemia detection. Translational Endocrinology Metabolism. 2012;3:47–87. doi: 10.1210/team.9781936704200.ch2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bain J., Plater L., Elliott M., Shpiro N., Hastie C.J., McLauchlan H. The selectivity of protein kinase inhibitors: a further update. The Biochemical Journal. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fryer L.G., Parbu-Patel A., Carling D. Protein kinase inhibitors block the stimulation of the AMP-activated protein kinase by 5-amino-4-imidazolecarboxamide riboside. FEBS Letters. 2002;531:189–192. doi: 10.1016/s0014-5793(02)03501-9. [DOI] [PubMed] [Google Scholar]
- 40.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J. Role of AMP-activated protein kinase in mechanism of metformin action. The Journal of Clinical Investigation. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdakov D., Jensen L.T., Alexopoulos H., Williams R.H., Fearon I.M., O'Kelly I. Tandem-pore K+ channels mediate inhibition of orexin neurons by glucose. Neuron. 2006;50:711–722. doi: 10.1016/j.neuron.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Hao L., Sheng Z., Potian J., Deak A., Rohowsky-Kochan C., Routh V.H. Lipopolysaccharide (LPS) and tumor necrosis factor alpha (TNFα) blunt the response of Neuropeptide Y/Agouti-related peptide (NPY/AgRP) glucose inhibited (GI) neurons to decreased glucose. Brain Research. 2016;1648:181–192. doi: 10.1016/j.brainres.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burdakov D., Gerasimenko O., Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. Journal of Neuroscience. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marston O.J., Hurst P., Evans M.L., Burdakov D.I., Heisler L.K. Neuropeptide Y cells represent a distinct glucose-sensing population in the lateral hypothalamus. Endocrinology. 2011;152:4046–4052. doi: 10.1210/en.2011-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Routh V., Hao L., Santiago A.M., Sheng Z., Zhou C. Hypothalamic glucose sensing: making ends meet. Frontiers in Systems Neuroscience. 2014;8 doi: 10.3389/fnsys.2014.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dulce Madeira M., Ferreira-Silva L., Paula-Barbosa M.M. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and golgi study. The Journal of Comparative Neurology. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- 47.Shupnik M.A. Oestrogen receptors, receptor variants and oestrogen actions in the hypothalamic-pituitary axis. Journal of Neuroendocrinology. 2002;14:85–94. doi: 10.1046/j.0007-1331.2001.00744.x. [DOI] [PubMed] [Google Scholar]
- 48.Bryzgalova G., Gao H., Ahren B., Zierath J.R., Galuska D., Steiler T.L. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- 49.Genabai N.K., Briski K.P. Adaptation of arcuate insulin receptor, estrogen receptor-alpha, estrogen receptor-beta, and type-II glucocorticoid receptor gene profiles to chronic intermediate insulin-induced hypoglycemia in estrogen-treated ovariectomized female rats. Journal of Molecular Neuroscience. 2010;41:304–309. doi: 10.1007/s12031-009-9314-4. [DOI] [PubMed] [Google Scholar]
- 50.Davis S.N., Shavers C., Costa F. Gender-related differences in counterregulatory responses to antecedent hypoglycemia in normal humans. The Journal of Cinical Endocrinology and Metabolism. 2000;85:2148–2157. doi: 10.1210/jcem.85.6.6641. [DOI] [PubMed] [Google Scholar]
- 51.Gingerich S., Krukoff T.L. Activation of ERbeta increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology. 2008;55:878–885. doi: 10.1016/j.neuropharm.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 52.Lagrange A.H., Wagner E.J., Rønnekleiv O.K., Kelly M.J. Estrogen rapidly attenuates a GABAB response in hypothalamic neurons. Neuroendocrinology. 1996;64:114–123. doi: 10.1159/000127106. [DOI] [PubMed] [Google Scholar]
- 53.Rey-Roldán E.B., Bianchi M.S., Bettler B., Becu-Villalobos D., Lux-Lantos V.A., Libertun C. Adenohypophyseal and hypothalamic GABAB receptor subunits are downregulated by estradiol in adult female rats. Life Sciences. 2006;79:342–350. doi: 10.1016/j.lfs.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 54.Claret M., Smith M.A., Batterham R.L., Selman C., Choudhury A.I., Fryer L.G. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. Journal of Clinical Investigation. 2007;117:2325–2336. doi: 10.1172/JCI31516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohno D., Sone H., Tanaka S., Kurita H., Gantulga D., Yada T. AMP-activated protein kinase activates neuropeptide Y neurons in the hypothalamic arcuate nucleus to increase food intake in rats. Neuroscience Letters. 2011;499:194–198. doi: 10.1016/j.neulet.2011.05.060. [DOI] [PubMed] [Google Scholar]
- 56.Taib B., Bouyakdan K., Hryhorczuk C., Rodaros D., Fulton S., Alquier T. Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. Journal of Biological Chemistry. 2013;288:37216–37229. doi: 10.1074/jbc.M113.506238. [DOI] [PMC free article] [PubMed] [Google Scholar]