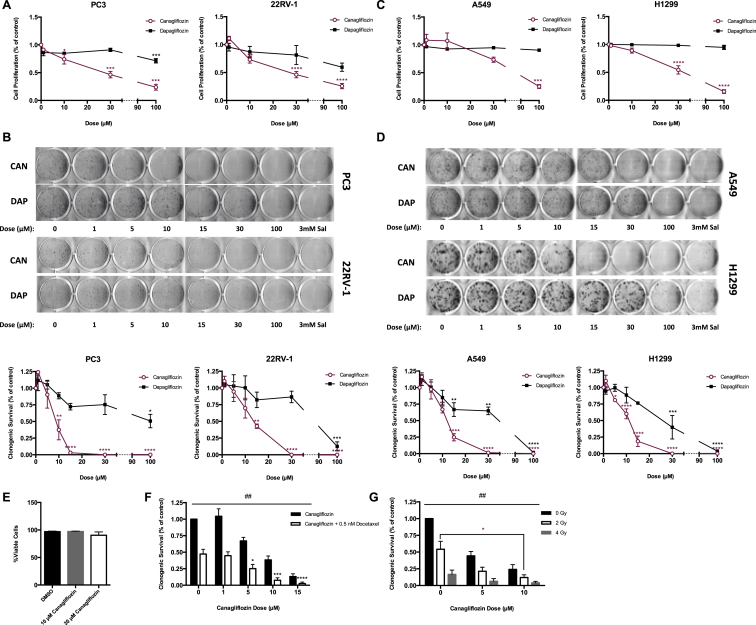
Figure 1.
Clinically effective concentrations of Canagliflozin inhibit the proliferation and clonogenic survival of cancer cells. (A) Cellular proliferation of prostate cancer cells (PC3 and 22RV-1) treated with Canagliflozin and Dapagliflozin and expressed relative to the vehicle controls for 72 h (n = 3, in quadruplicate). (B) Clonogenic survival of prostate cancer cells treated with Canagliflozin, Dapagliflozin or Salicylate and expressed relative to the vehicle controls. Representative images above and quantifications (n = 3, in triplicate) shown below. (C) Cellular proliferation of lung cancer cells (A549 and H1299) treated with Canagliflozin and Dapagliflozin and expressed relative to the vehicle controls for 72 h (n = 3, in quadruplicate). (D) Clonogenic survival of lung cancer cells treated with Canagliflozin, Dapagliflozin, or Salicylate and expressed relative to the vehicle controls. Representative images above and quantifications (n = 3, in triplicate) shown below. (E) Percentage of viable PC3 cells treated with Canagliflozin or vehicle over 72 h (n = 5, in duplicate). (F) Clonogenic survival of PC3 cells treated with Canagliflozin as a single agent or in combination with 0.5 nM Docetaxel (n = 3, in triplicate). (G) Clonogenic survival of PC3 cells treated with Canagliflozin as a single agent or in combination with 0, 2 or 4 Gy of radiation (n = 3, in triplicate). Results are expressed as the mean and standard error of the mean (SEM). Vehicle versus treatment * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001 by one-way ANOVA for A–D, by two-way ANOVA for F–G. Single versus combination treatment ## = p < 0.01 by two-way ANOVA for F–G.