Fig. 2.
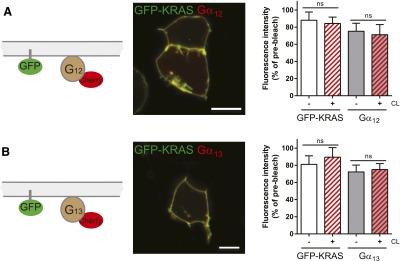
Nonreceptor control ensures that the mobile fraction of Gα12/13-mCherry is not affected by chemical surface crosslinking in the absence of FZD4. HEK293 cells expressing farnesylated GFP-KRAS, untagged βγ subunits, and N-terminally tagged Gα12-mCherry were used for a dcFRAP assay using chemical surface crosslinking (CL) with Sulfo-NHS-LC-LC-biotin and avidin as described in Fig. 1. CL affected neither the mobile fraction of GFP-KRAS nor that of Gα12-mCherry (A) or Gα13-mCherry (B). The figure includes a schematic presentation clarifying the experimental setup, a confocal micrograph showing HEK293T cells coexpressing GFP-KRAS with the Gα12-mCherry (A) or Gα13-mCherry (B; size bars = 10 µm), fluorescence intensity curves before (gray) and after (red) CL for both GFP-KRAS and Gα12/13-mCherry, and a bar graph summarizing the mobile fractions of GFP-KRAS and the Gα subunit under each experimental condition. The data verify that the decrease in G protein mobile fraction observed for Gα12/13-mCherry in the presence of FZD4-GFP is not evoked by CL of endogenously expressed receptors. Error bars provide the S.E.M. ns, not significant. ***P < 0.001. Bar graph summarizes measurements from at least four independent experiments, each including data from several individual cells.
