Figure 2.
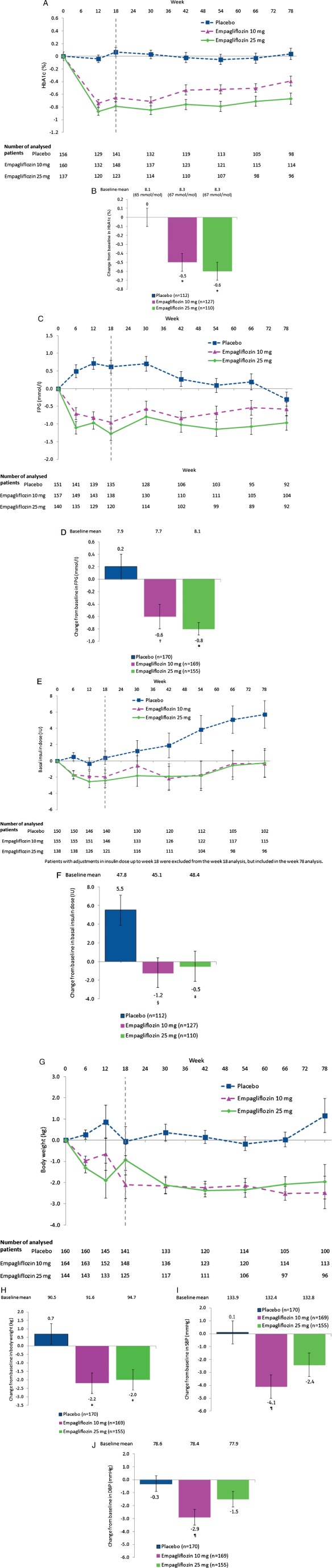
Effect of empagliflozin on efficacy parameters at week 78. (A) Glycated haemoglobin (HbA1c) over time [mixed model repeated measures (MMRM), full analysis set (FAS), observed cases (OC)]; (B) change from baseline in HbA1c [analysis of covariance (ancova), FAS week 78 completers, last observation carried forward (LOCF) imputation]; (C) fasting plasma glucose (FPG) over time (MMRM, FAS, OC); (D) change from baseline in FPG (ancova, FAS, LOCF); (E) basal insulin dose over time (MMRM, FAS, OC); (F) change from baseline in basal insulin dose at week 78 (ancova, FAS‐78 completers, LOCF); (G) body weight over time (MMRM, FAS, OC); (H) change from baseline in body weight (ancova, FAS, LOCF); (I) change from baseline in SBP (ancova, FAS, LOCF); (J) change from baseline in DBP (ancova, FAS, LOCF). Data are mean ± standard error (s.e.) at baseline and adjusted mean ± s.e. on treatment. *p < 0.001 vs placebo; †p = 0.005 vs placebo; §p = 0.002 vs placebo; ‡p = 0.009 vs placebo; ¶p = 0.004 vs placebo.
