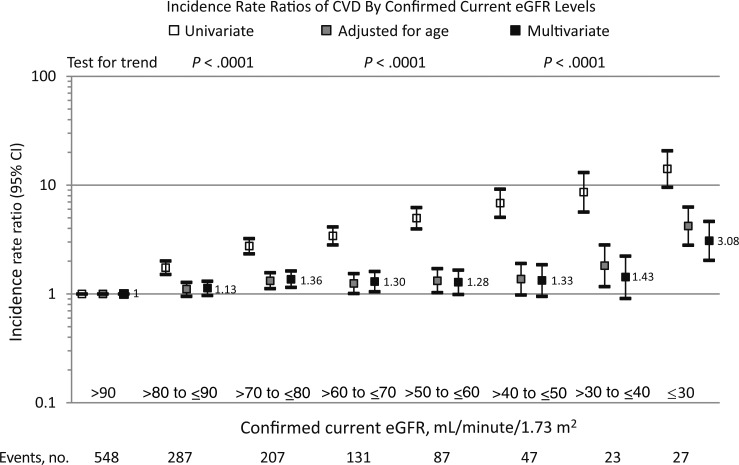
Figure 3.
Cardiovascular disease (CVD) incidence rate ratios by confirmed current estimated glomerular filtration rate (eGFR). Multivariate analysis adjusted for age, sex, ethnicity, Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study enrollment cohort, nadir CD4+ T-cell count, mode of human immunodeficiency virus (HIV) acquisition group, and family history of CVD at baseline. Time-updated variables include hepatitis B virus/hepatitis C virus coinfection, HIV RNA level, CD4+ T-cell count, prior AIDS, hypertension, diabetes, confirmed eGFR strata, smoking status, dyslipidemia, prior CVD, exposure to antiretroviral drugs fitted as cumulative use (to zidovudine, didanosine, zalcitabine, stavudine, lamivudine, emtricitabine, tenofovir disoproxil fumarate, abacavir, efavirenz, nevirapine, indinavir, saquinavir, ritonavir, nelfinavir, (fos)ampreavir, atazanavir, and darunavir) and current use (zidovudine, didanosine, zalcitabine, lamivudine, stavudine, emtricitabine, tenofovir disoproxil fumarate, and abacavir). Abbreviation: CI, confidence interval.