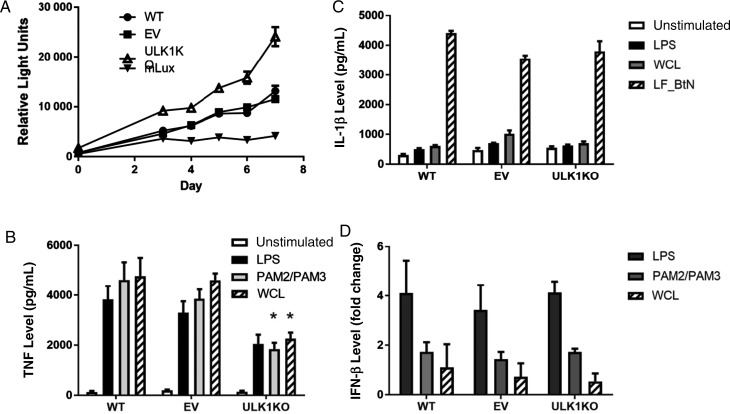
Figure 2.
Differential responses in ULK1-deficient monocyte derived macrophages. U937 cells with either the ULK1 knockout (KO), the empty-vector CRISPR control (EV), or wild type (WT). A, Mycobacterium tuberculosis replication in ULK1-deficient monocyte-derived macrophages. KO, EV, and WT cells infected with luciferase-expressing M. tuberculosis at a multiplicity of infection (MOI) of 10. A separate set of wells was included with luciferase-expressing M. tuberculosis (mLux) in the absence of U937 cells as a control for background luciferase expression. Relative light units were measured at the indicated time points. Each point represents the mean of 6 separate wells. P = .004 by the Wilcoxon rank-sum test on days 3–7 for ULK1-deficient cells as compared to cells with EV or WT. B, After stimulation with PAM2/PAM3 (250 ng/mL each), lipopolysaccharide (LPS; 100 ng/mL), and M. tuberculosis whole-cell lysate (WCL; 25 µg/mL) for 24 hours, tumor necrosis factor (TNF) was measured by enzyme-linked immunosorbent assay (ELISA). ULK1-deficient cells produced significantly less TNF in response to PAM2/PAM3 (*P = .004 by the Student t test) and WCL (P < .001) as compared to cells with WT or EV. C, After stimulating the inflammasome with Bacillus thailandensis needle protein fused to lethal factor for cytoplasmic delivery (LF_Btn), interleukin 1β (IL-1β) secretion was measured by ELISA . D, After stimulation with PAM2/PAM3 (250 ng/mL, each), LPS (100 ng/mL), and WCL (25 µg/mL) for 24 hours, interferon β (IFN-β) was measured by reverse transcription–polymerase chain reaction. Linearized transcription values are expressed as fold change as compared to unstimulated cells. Graph includes merged data from 2 independent experiments.