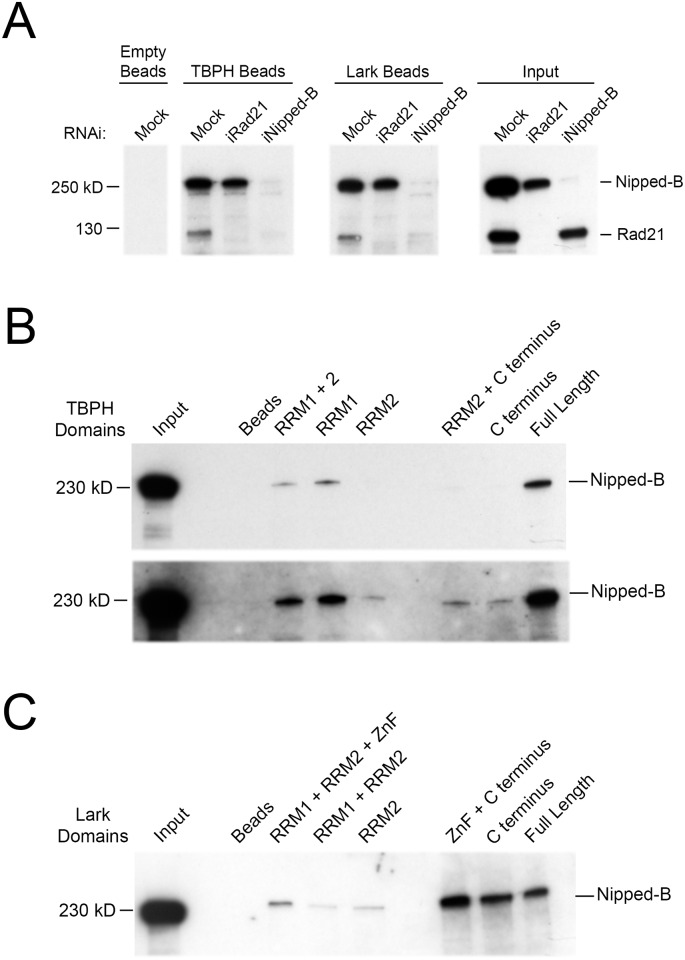
Fig 8. TBPH and Lark interact with Nipped-B.
(A) The western blots show the binding of Nipped-B and cohesin (Rad21) in BG3 cell nuclear extracts to NTA-Zn2+ agarose beads with immobilized His6-SUMO-TBPH and -Lark fusion proteins probed with both Nipped-B and Rad21 antibodies. The western on the far right (Input) shows the Nipped-B and Rad21 proteins in the BG3 cell nuclear extracts used for the binding experiments. Nuclear extracts were prepared from control (Mock) cells and cells depleted for Rad21 and Nipped-B by RNAi. The results shown for mock nuclear extract are representative of five independent experiments. The results shown for nuclear extracts depleted for Rad21 and Nipped-B are representative of two technical replicates. S12 Fig shows that native TBPH and Lark co-immunoprecipitate with Nipped-B from nuclear extracts, that Lark and TBPH do not co-precipitate, and that pre-treatment of BG3 nuclear extract with ribonucleases does not prevent binding of Nipped-B to TBPH and Lark beads. (B) Western blot of Nipped-B binding to the indicated TBPH fragments and full-length TBPH to map the protein domains interacting with Nipped-B. The bottom panel is a longer exposure of the same blot to show low levels of Nipped-B binding to some fragments. The fragments are shown in S10 Fig. Most of the Nipped-B binding occurs with the RRM1 domain of TBPH. The blots shown are representative of three independent experiments. (C) Western blot of Nipped-B binding to the indicated fragments of Lark and full-length Lark. The fragments are shown in S10 Fig. The blot shown is representative of three independent experiments.