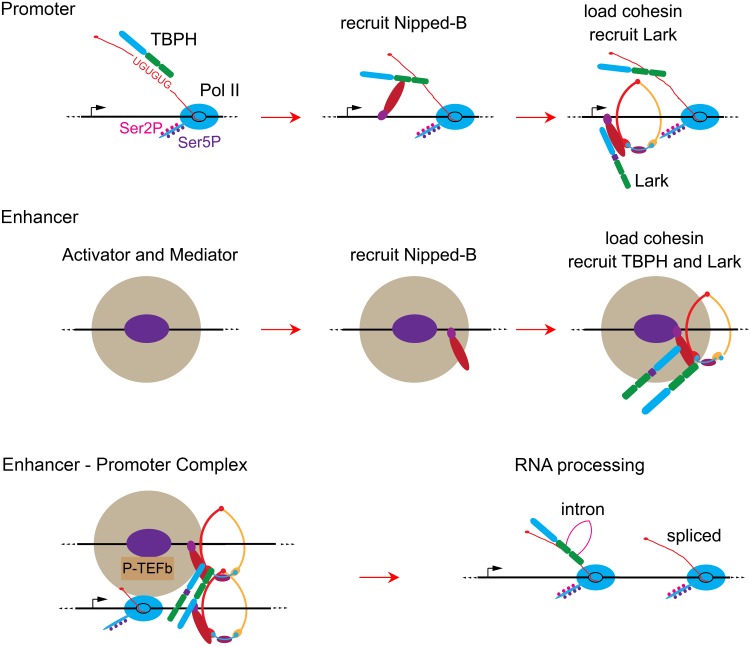
Fig 9. Hypothetical models for roles of TBPH and Lark in Nipped-B and cohesin binding to genes and enhancers, enhancer-promoter interactions, and processing of nascent RNA transcripts.
At promoters (top row) we posit that TBPH binds to UG repeats in the first nascent transcripts produced by elongating Pol II (Ser2P / Ser5P) when a gene is initially activated. TBPH then recruits Nipped-B, which interacts with DNA, loads cohesin and recruits the Lark RNA-binding protein. At enhancers (middle row) activator proteins (purple oval) recruit Mediator (large tan circle) and Nipped-B. Nipped-B then loads cohesin and recruits TBPH and Lark, and TBPH stabilizes binding of the complex. The protein complexes at the enhancers and promoters form enhancer-promoter complexes (bottom row) that are stable for hours even in the absence of new transcription initiation. TBPH contributes to their stability, while Lark destabilizes cohesin and Nipped-B binding, particularly to the promoter. P-TEFb in the enhancer-promoter complex phosphorylates paused Pol II and the pausing factors (not depicted), leading to transcriptional elongation (lower right). Some TBPH and Lark present in the enhancer-promoter complex binds to the nascent RNA produced by the elongating polymerase and can facilitate RNA processing. For example, they can regulate intron removal by splicing as depicted in the lower right diagram, in addition to other processes.