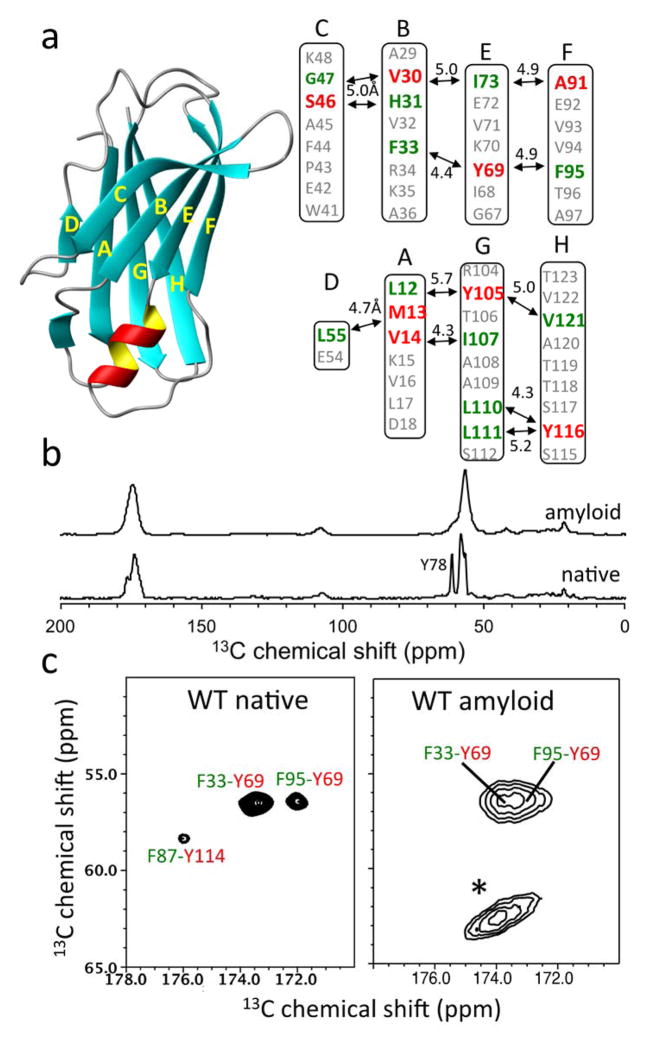
Figure 2.
(a) Ribbon diagram representation of TTR monomer and labeling schemes for the solid-state NMR experiments. The 13CO (green) and 13Cα (red) carbons of the amino acids are labeled if the internuclear distance in the native TTR tetramer are between 4 and 6 Å. (b) 13C CPMAS spectra of the native and amyloid states of TTR prepared from 13CO-Phe and 13Cα-Tyr labeled TTR. (c) 2D PDSD spectra of the native and amyloid states of TTR with a contour level of 1.5 % with respect to the diagonal peak obtained using a mixing time of 500 ms at a 1H frequency of 830 MHz. * denotes spinning sidebands. For the solid-state NMR experiments, the native tetrameric TTR at pH 7.3 was precipitated using 90 % ammonium sulfate and dried under Ar gas. Both dried native and amyloid samples were rehydrated with 5 μL water.