Figure 1.
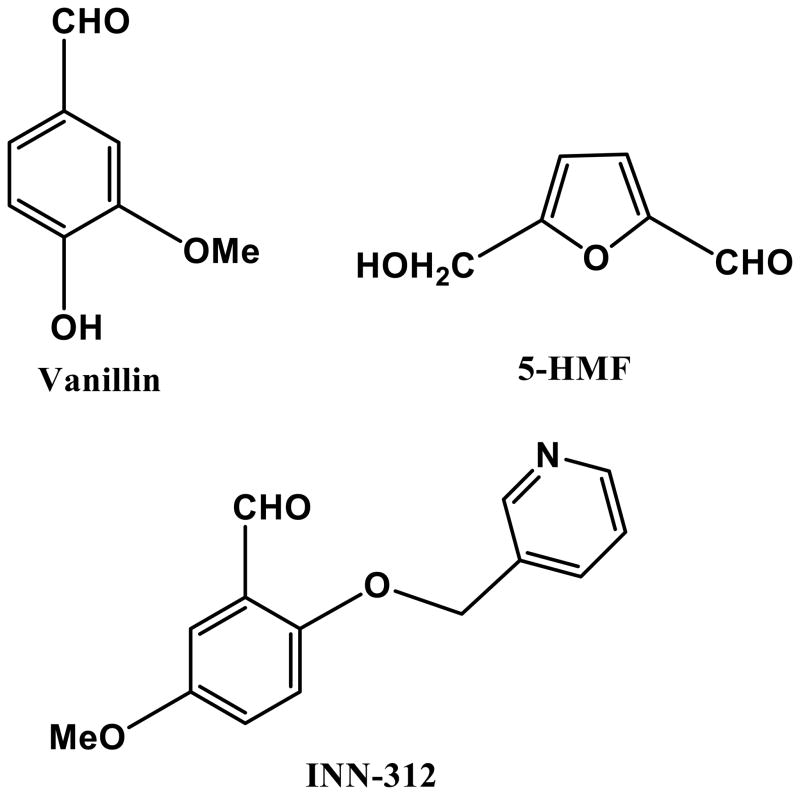
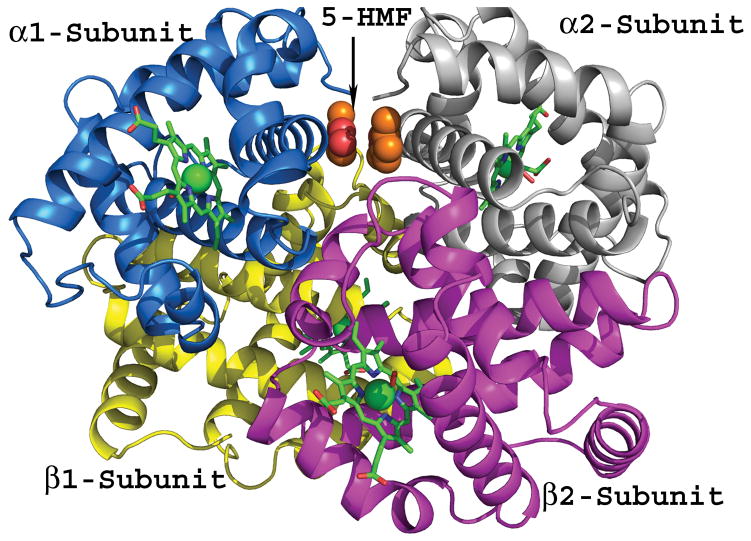
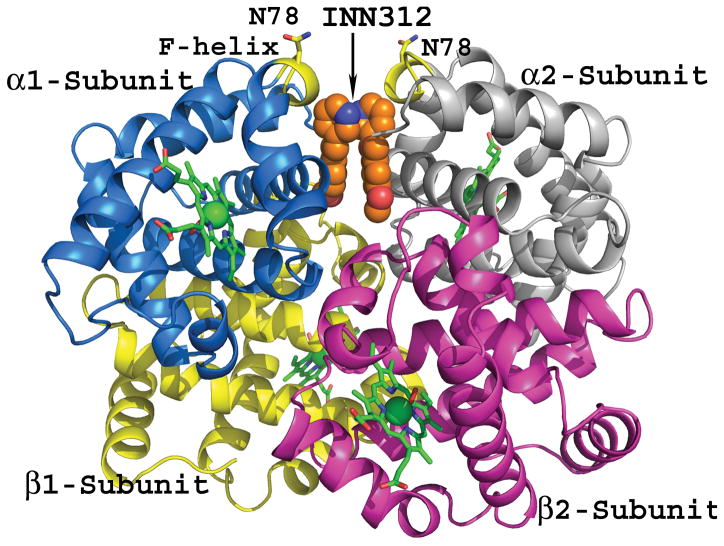
Chemical structures of aromatic aldehydes and their complexes with liganded haemoglobin. (A) Structures of aromatic aldehydes. (B) Binding of 5-hydroxymethylfurfural (5-HMF; orange) in a symmetry-related fashion at the α-cleft of liganded Hb, and through a series of inter-subunit hydrogen-bond and/or hydrophobic interactions, stabilize the R-state conformation. (C) Binding of a pyridyl derivative of vanillin, INN-312 (orange) in a symmetry-related fashion at the α-cleft of liganded Hb, which leads to stabilization of the relaxed state conformation. Additionally, the pyridine moiety of INN-312 makes hydrophobic interactions with the F-helix, perturbing the inter-strand polymer contact involving Asn78, and contributing to the anti-sickling activity of the compound.