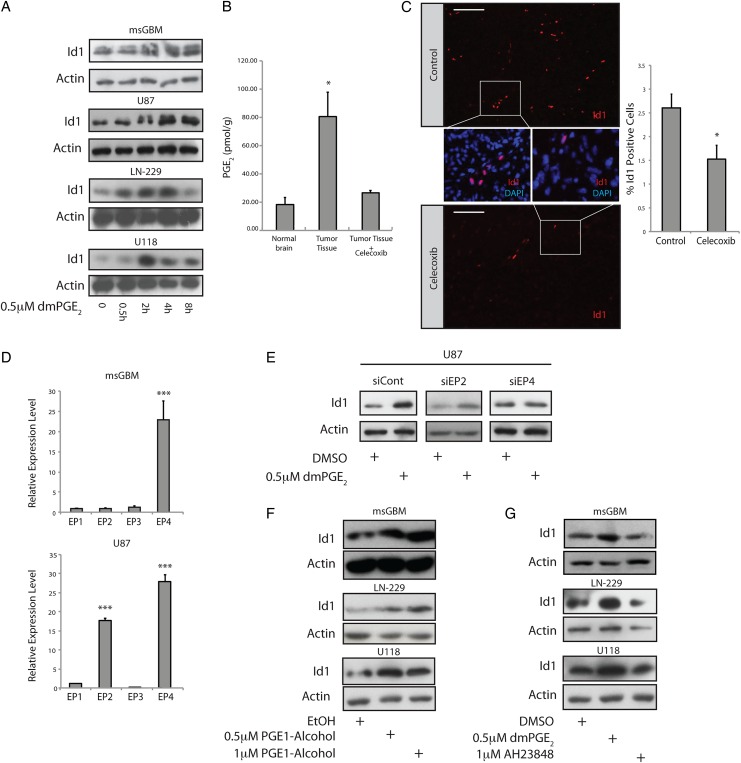
Fig. 2.
The EP4 receptor is responsible for PGE2-mediated induction of Id1. (A) Treatment of primary mouse glioblastoma (GBM) cultures or human U87, LN-229, or U118 glioblastoma cells with 0.5 µM dmPGE2 led to time-dependent induction of Id1 protein. (B) GBMs were initiated in Nestin-tva, Arf −/− mice at day zero via stereotactic injection RCAS-PDGFB virus. At day 23, tumor-bearing mice were treated with 100 mg/kg/day celecoxib or vehicle control (n = 4 per group) by gavage for 4 days, followed by sacrifice and tumor harvest. Harvested tissue was subjected to mass spectrometry to quantify PGE2 levels in normal brain versus GBM with and without celecoxib treatment. *, P < .05, Error bars represent mean ± SEM. (n = 4). (C) Immunohistological analysis of Id1 in tumors derived from mice treated with vehicle (n = 4) or celecoxib (n = 3). Three non–overlapping fields were quantitated for each tumor. Scale bar = 0.1 mm. Error bars represent mean ± SEM. (n = 3 or 4). *, P < .05. (D) To identify the relevant PGE2 receptor in GBM cells, we performed quantitative real-time (qRT) PCR for the 4 EP receptors in mouse GBM cells (upper panel) and human U87 cells (bottom panel). The level of EP4 was notably higher than EP1-3, when EP1 was used as the point of comparison in both cell types, while U87 cells also displayed elevated EP2. Error bars represent mean ± SEM. (n = 3). ***, P < .001. (E) EP2/4 receptor expression was knocked down in human U87 GBM cells via transient transfection with specific siRNAs. Id1 upregulation in response to dmPGE2 treatment was inhibited specifically with EP4 knockdown. (F) Treatment of primary mouse GBM cells or human LN-229 or U118 glioblastoma cell lines with the EP4 receptor agonist PGE1-alcohol resulted in a dose-dependent increase in Id1 protein levels. (G) Treatment with an EP4 antagonist (AH23848) effectively blocked dmPGE2-mediated induction of Id1.