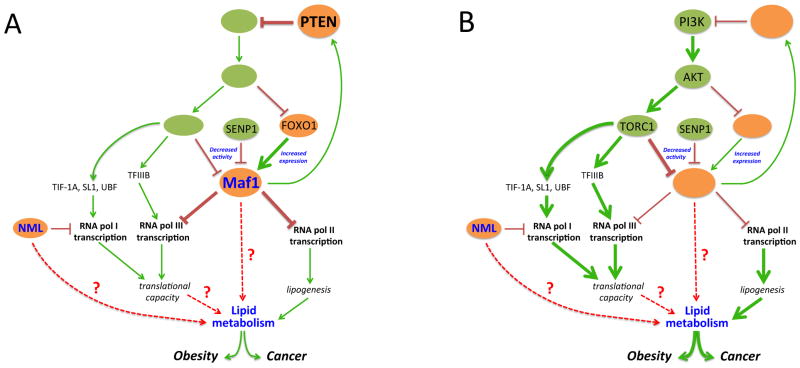
KEY FIGURE, Figure 1.
PTEN coordinately regulates RNA and lipid metabolism through Maf1
PTEN acts through its downstream signaling targets to repress transcription from all three nuclear RNA polymerases. PTEN controlled signaling events target Maf1, a global repressor of RNA pol III-dependent transcription and a selective repressor of RNA pol II-mediated transcription. TORC1 and SENP1 regulate PTMs on Maf1 to negatively regulate its activity while FOXO1 positively regulates Maf1 expression. Maf1-mediated changes in gene expression coordinate the repression of both lipid biogenesis and the translational capacity of cells. In mouse models, the loss of either NML or Maf1, negative regulators of RNA pol I- and pol III-dependent transcription, respectively, leads to transcription induction and dysregulation of lipid metabolism. Maf1 represents a novel molecular link between RNA and lipid metabolism that may explain the established association between obesity and cancer. However, more work is needed to understand how Maf1 and NML contribute to lipid homeostasis and whether Maf1-mediated repression of RNA pol III-mediated transcription contributes to lipid metabolism. (A) In cells with functional PTEN, PI3K/AKT/TORC1 signaling is inhibited while FOXO1 is activated. Inactivation of mTORC1 limits the expression of RNA pol I and III transcribed genes by reducing the activity of their transcription components. The inability of TORC1 to phosphorylate Maf1 leads to enhanced Maf1 activity while the activation of FOXO1 leads to enhanced Maf1 expression. Maf1 also induces the expression of PTEN, leading to further inactivation of AKT. The overall increase in cellular Maf1 activity coordinates repression of its RNA pol II and III target genes to limit translational capacity and lipogenesis. Maintaining repression of these processes may suppress the development of cancer and metabolic diseases such as obesity. (B) Inactivation or loss of PTEN results in constitutive activation of PI3K and AKT. The resultant activation of mTORC1 leads to induction of both RNA pol I- and III-dependent transcription through enhanced activity of transcription factors. The activation of TORC1 also causes a reduction in Maf1 activity while the inactivation of FOXO1 results in a decrease in Maf1 expression. This overall decrease in Maf1 leads to a derepression of Maf1 targeted RNA pol II and III transcribed genes leading to enhanced translational capacity and lipogenesis. Dysregulation of these processes and altered RNA and lipid metabolism may contribute to the development of obesity and cancer.