Fig. 1. Schematic Representation of the Cytoprotective and Cytototxic Anticipatory UPR Pathways.
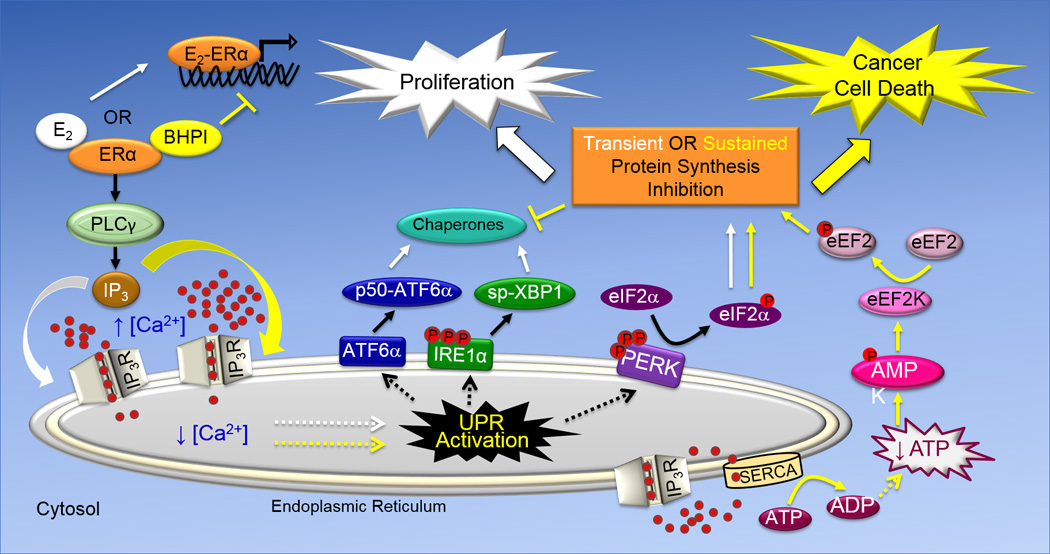
The UPR consists of three arms, ATF6α, IRE1α and PERK that are named after proteins embedded in the membrane of the endoplasmic reticulum. While activation of the ATF6α and IRE1α arms of the UPR leads to induction of chaperones that increase protein folding capacity, activation of the PERK arm of the UPR leads to reduced protein production. ATF6α activation leads to translocation of the transmembrane protein p90-ATF6α from the EnR to the Golgi apparatus, where it encounters proteases that liberate the N-terminal fragment of ATF6α (p50-ATF6α). p50-ATF6α is a transcription factor that increases the protein-folding capacity of the EnR by inducing EnR-resident chaperones, including BiP. The transmembrane protein IRE1α is a nuclease. Oligomerization and phosphorylation activates IRE1α. Activated IRE1α removes an intron from full-length XBP1 mRNA, producing spliced (sp)-XBP1 mRNA. sp-XBP1 is a transcription factor that increases the protein-folding capacity of the EnR and turnover of misfolded proteins by inducing EnR resident-chaperones. Oligomerization and autophosphorylation activate the transmembrane kinase PERK. Activated p-PERK phosphorylates eukaryotic initiation factor 2α (eIF2α), leading to inhibition of protein synthesis and a reduction in the endoplasmic reticulum protein folding load. E2 and BHPI bind at distinct sites on ERα. In the anticipatory UPR (shown on the left), E2 and BHPI act via ERα to activate a pathway that results in opening of EnR IP3R calcium channels allowing efflux of calcium stored in the lumen of the EnR into the cytosol. This activates the UPR. In ERα containing cancer cells, strong and sustained opening of the EnR IP3R calcuim channels by BHPI sets up a futile cycle of ATP-dependent pumping of calcium into the EnR by SERCA pumps and leakage back into the cytosol through the open IP3R channels (lower right). This depletes intracellular ATP, activating the energy sensor AMPK (AMP kinase). Elevated intracellular calcium and activated AMPK phosphorylate and activate eukaryotic elongation factor 2 (eEF2) kinase. eEF2 kinase phosphorylates and inactivates eEF2. This inhibits protein synthesis at a second site, resulting in strong and sustained toxic inhibition of protein synthesis by BHPI.
