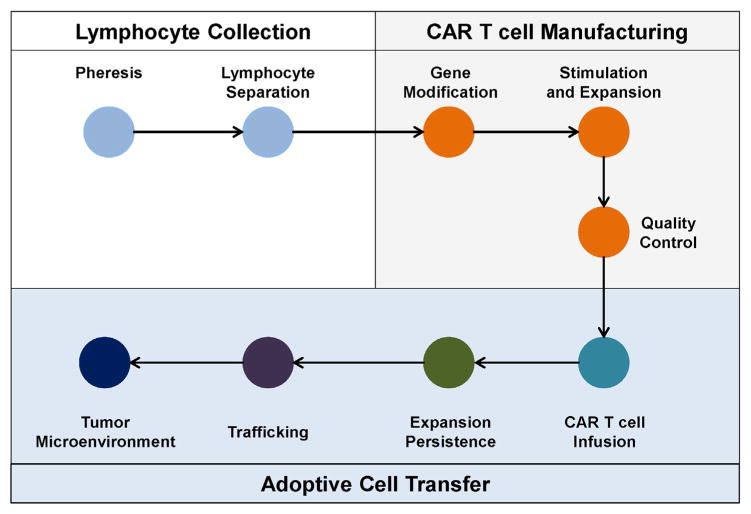
Fig. 3. CAR T cell efficacy in solid malignancies is dependent on multiple steps.
CAR T cell therapy is a multi-step process involving (i) pheresis to collect peripheral blood leukocytes (i.e. leukapheresis) and elutriation to isolate lymphocytes from the peripheral blood of patients (Lymphocyte Collection); (ii) manufacturing of CAR T cells including gene modification of T cells to express the CAR, stimulation/expansion of gene-modified T cells in vitro, and quality control measures to evaluate CAR expression levels, T cell quality, and infectious contamination (CAR T cell Manufacturing); and (iii) the adoptive transfer of T cells to patients which then must expand, persist, traffick to tumors, and mediate effector anti-tumor activity within the tumor microenvironment (Adoptive Cell Transfer). Each of these steps is critical to the efficacy of CAR T cell therapy in solid malignancies.