Figure 2.
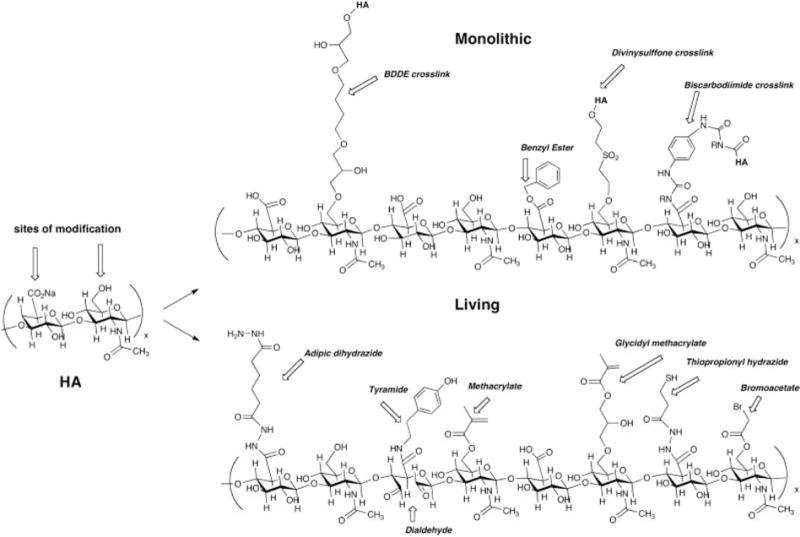
Sample chemical structures of monolithic (top) and living (bottom) chemical modifications of hyaluronic acid. A hypothetical composite structure illustrates selected primary modifications: adipic dihydrazide for use in further crosslinking via acrylamide or hydrazone linkages; butane-1,4-diol diglycidyl ether, a prototypical monolithic crosslinker for HA; tyramide for peroxidase crosslinking; dialdehyde obtained by periodate oxidation; methacrylate on primary 6-hydroxyl group; benzyl ester; glycidyl methacrylate; thiopropionyl hydrazide from DTPH modification; bromoacetate; an unmodified disaccharide unit for comparison.[66] Reprinted with permission from Burdick, J. A. and Prestwich, G. D. Adv. Mater. (2011) 23, H41.Copyright (2011) Wiley.
