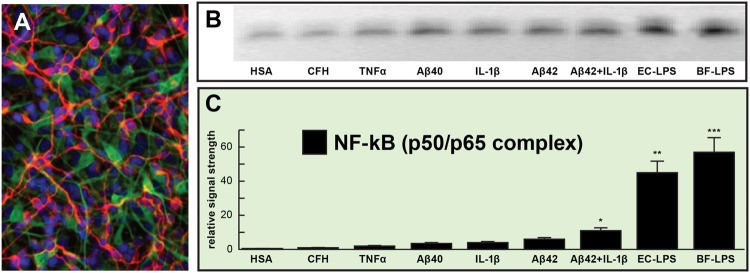
FIGURE 1.
Relative induction of NF-kB (p50/p65)-DNA binding in pro-inflammatory factor and lipopolysaccharides (LPS)-treated primary human neuronal-glial (HNG) co-cultures. (A) HNG cells in primary co-culture for 1.5 weeks; HNG cells were stained with a neuron-specific β-tubulin III (red fluorescence λ max~650 nm; anti-βTUBIII antibody, Sigma-Aldrich, St Louis, MO, USA); an antibody against glial fibrillary acidic protein (GFAP; green fluorescence; λ max ~510 nm; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and DAPI nuclear stain (blue fluorescence; λ max~470 nm; Thermo Fisher Scientific, Waltham, MA, USA); 20×; (B) induction of the pro-inflammatory transcription factor NF-kB (p50/p65 activation complex) by various physiologically relevant, pro-inflammatory factors all at equal dosage (25 nM); NF-kB abundance was measured by NF-kB-DNA binding assay (a measure of NF-kB activation and binding to NF-kB-DNA recognition sequences) onto a 36 nucleotide end-labeled double stranded DNA fragment containing the canonical human NF-kB (p50/p65) recognition sequence 5′-GGGGACTTTCCC-3′ as previously described (Lukiw and Bazan, 1998; Lukiw et al., 2008; Devier et al., 2015; Clement et al., 2016); a scrambled control nucleotide containing no such NF-kB recognition sequence showed NF-kB-DNA binding activity (data not shown); (C) data from gel bands in panel (B) quantified in bar graph format; note robust induction of the NF-kB (p50/p65 complex) by Escherichia coli lipopolysaccharide (EC-LPS; LPS from Escherichia coli 0111:B4; Sigma L3012, St Louis, MO, USA) or B. fragilis lipopolysaccharide (BF-LPS; prepared by methods previously published; Eidhin and Mouton, 1993) that was ~45- to ~55-fold higher than that of the control human serum albumen (HSA) protein, and was fivefold to sevenfold higher than the combination of the pro-inflammatory Aβ42 peptide and IL-1β together (at 25 nM each); complex mixtures of microbiome bacterial LPS on NF-kB induction might be expected to be additive or synergistic; HSA = (control) human serum albumen; CFH = complement factor H; TNFα = tumor necrosis factor alpha (cachectin); IL-1β = interleukin 1-beta; Aβ40, Aβ42 = amyloid beta peptide, 40 and 42 amino acids in length; EC-LPS, BF-LPS = E. coli, Bacteroides fragilis lipopolysaccharide; error bars represent one standard error of the mean; N = 4; *p < 0.05; **p < 0.01; ***p < 0.001, ANOVA.