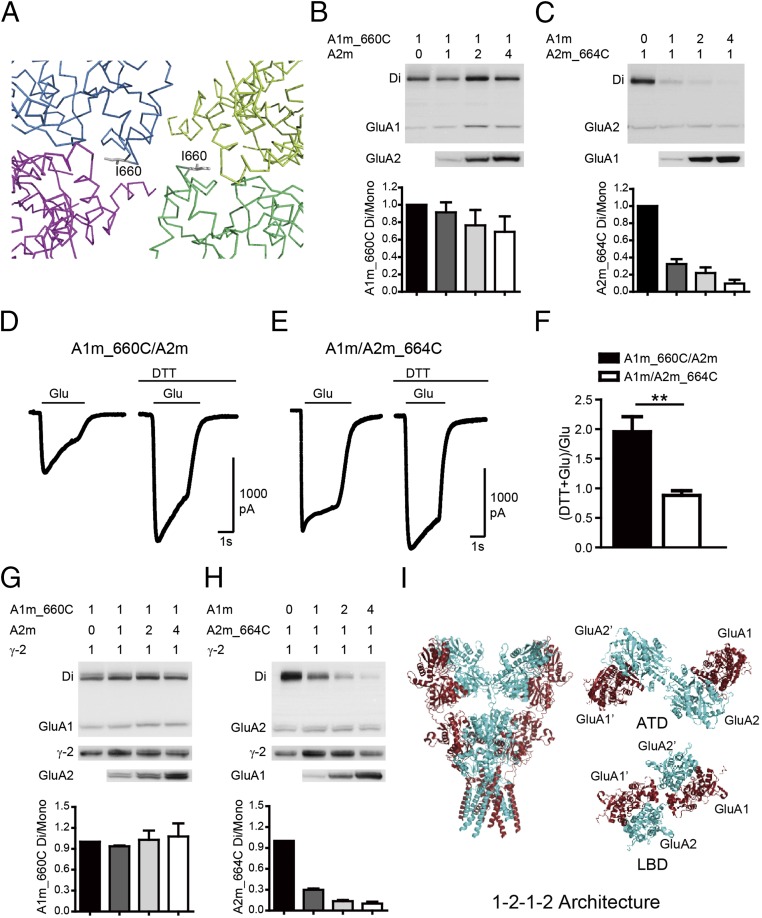
Fig. 2.
GluA1 and GluA2 have preferred positions in tetrameric assemblies. (A) Stereoview showing cysteine mutants designed to cross A and A′ subunits in a LBD tetramer assembly, with each subunit colored the same as for the GluA1 tetramer shown in Fig. 1. (B) The GluA1m_I660C mutation showed spontaneous disulfide-bond formation. Quantifications of ratio between the dimer to the monomer band indicated GluA1 in the A conformation. (C) Spontaneous cross-link in GluA2m_I664C mutation was diminished by coexpression with GluA1m. Quantifications of ratio between the dimer to the mono band indicated GluA2 in the B conformation. (D–F) The glutamate-induced currents were enhanced by DTT in the GluA1m_I660C/GluA2m channel (n = 8), but not in the GluA1m/GluA2m_I664C channel (n = 8). Values are means ± SEMs. **P < 0.01 (t test). (G and H) AMPAR subunits TARP γ-2 did not change heterotetrameric AMPAR assembly. (I) Model of heteromeric AMPARs with a preferred 2:2 stoichiometry and assemble into 1–2–1–2 conformation.