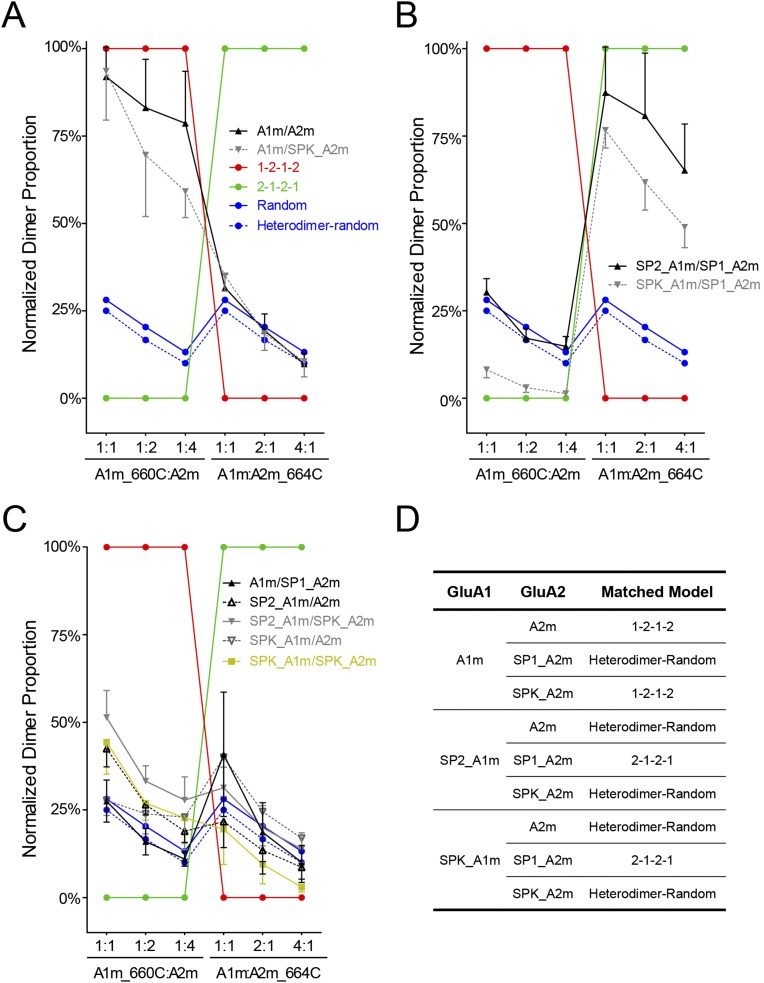
Fig. S9.
Theoretical model of spatial assembly for the GluA1/A2 heterotetramer. Cysteine residues were introduced at the interface between LBD dimers. The Cys-tagged subunit was coexpressed with an untagged pattern with variable ratios. The possible position of the subunits was predicted with three models (i.e., preferred 1–2–1–2, preferred 2–1–2–1, and random assembly). A fourth model assumed that GluA1/A2 preferred to form heterodimers and then assembled into tetramers in a random manner. The experimental data were compared with those models. (A) GluA1m/A2m and GluA1m/SPK_GluA2m had similar cross-linking patterns. The dimer bands of GluA1 subunits were relatively stable with the coexpression of one to four times GluA2m. The cross-linking patterns are closely represented by the 1–2–1–2 model. (B) SP1_GluA1m/SP1_GluA2m and SPK_GluA1m/SP1_GluA2m had similar cross-linking pattern that can be best explained with the 2–1–2–1 model. (C) Under five conditions, the GluA1/A2 cross-linking pattern were close to the hetero-random model, where heteromeric dimers were preferred to form before random assembly into tetramers. (D) Summary of how SPs change the spatial assembly pattern.