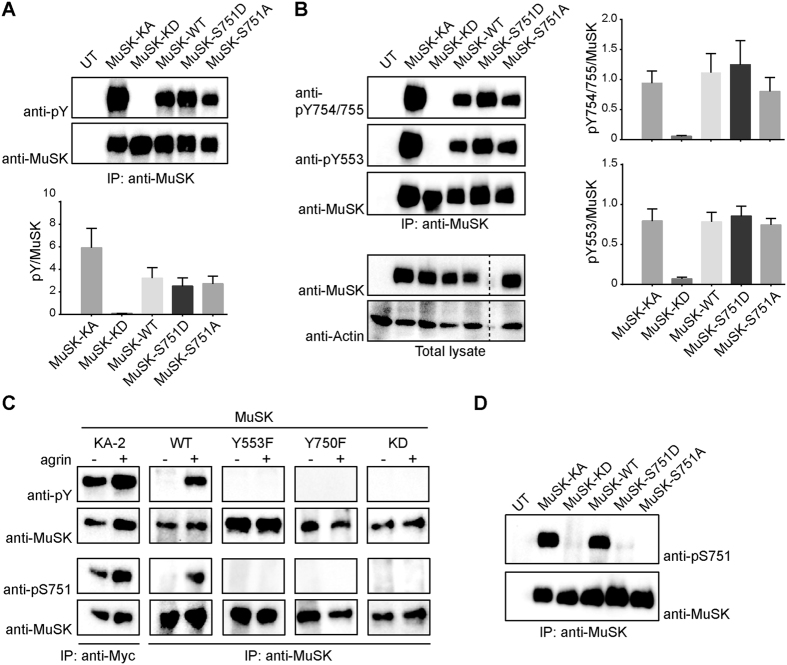
Figure 4. S751 does not regulate autoactivation of MuSK in heterologous cells and phosphorylation of S751 is dependent on MuSK kinase activity.
(A) HEK 293T cells were transiently transfected with wild-type and mutant MuSK constructs resulting in autoactivation of MuSK kinase. Cell lysates were subjected to immunoprecipitation (IP) using anti-MuSK antibodies and immunoblotted with anti-phospho-tyrosine (pY) and anti-MuSK antibodies, respectively. Wild-type MuSK (WT) is strongly tyrosine phosphorylated. Kinase-dead MuSK (KD) lacks tyrosine phosphorylation whereas phosphorylation of kinase-active MuSK (KA) is further increased compared to wild-type. S751 mutants are similarly autoactivated as MuSK wild-type. Values are presented as the mean ± S.E.M. (Kruskal-Wallis with Dunn’s multiple comparison test, n = 5). (B) HEK 293T cells were transiently transfected with wild-type and mutant MuSK constructs resulting in autoactivation of MuSK kinase. Cell lysates were subjected to immunoprecipitation (IP) using anti-MuSK antibodies and immunoblotted using antibodies against MuSK pY553, MuSK pY754/755 and MuSK, respectively. Total lysates were analysed with antibodies against MuSK and Actin, respectively. Phosphorylation of juxtamembrane Y553 and Y754, Y755 of the kinase loop occur at similar level in S751 mutant MuSK compared to MuSK-wild-type. Values are presented as the mean ± S.E.M. (Kruskal-Wallis with Dunn’s multiple comparison test, n = 5). (C) Muscle cells expressing MuSK kinase mutants were stimulated with agrin (+, A4B8; −, A0B0) and cell lysates were subjected to immunoprecipitation (IP) using anti-MuSK or anti-Myc antibodies. Samples were analysed by immunoblotting using antibodies against phospho-tyrosine (PY), MuSK pS751 and MuSK, respectively. Kinase-dead MuSK (KD), MuSK Y750, 754, 755F (Y750F) and MuSK Y553F are unresponsive to agrin. Wild-type MuSK (WT) is phosphorylated in response to agrin and kinase-active MuSK (KA-2) is highly phosphorylated in the presence and absence of agrin. Note that phosphorylation of S751 depends on MuSK tyrosine phosphorylation. (D) HEK 293T were transiently transfected with wild-type and mutant MuSK constructs resulting in autoactivation of MuSK kinase. Cell lysates were subjected to immunoprecipitation (IP) using anti-MuSK antibodies and immunoblotted with anti-MuSK pS751 and anti-MuSK antibodies, respectively. Phosphorylation of S751 is only detected upon autoactivation of the MuSK kinase. Full-length blots are presented in Supplementary Fig. S9.