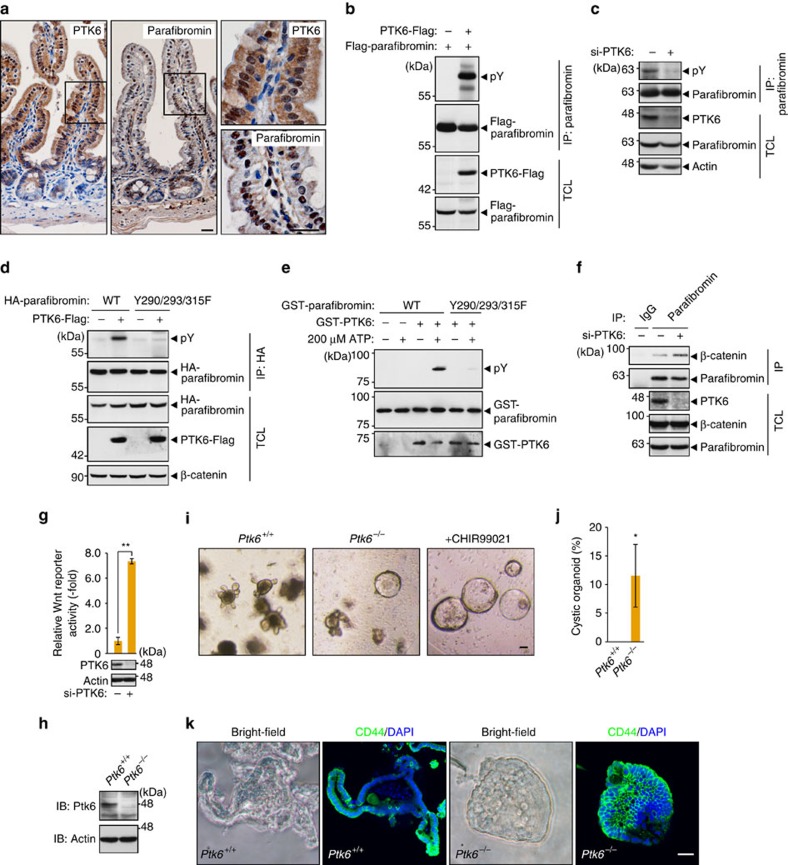
Figure 5. PTK6 phosphorylates parafibromin and impairs its scaffold function.
(a) Immunohistochemistry of the mouse small intestine confirming the co-expression of PTK6 and parafibromin. The right panels show higher-magnification images. Scale bars, 20 μm. (b,c) PTK6 regulates parafibromin tyrosine phosphorylation. AGS cells were transfected with the indicated vectors (b) or si-PTK6 (c). Total cell lysates (TCLs) were sequentially immunoprecipitated (IP) with the indicated antibodies. (d) Y290/293/315F mutations abolish parafibromin phosphorylation by PTK6. AGS cells were transfected with the indicated vectors. TCLs were sequentially immunoprecipitated (IP) with the indicated antibodies and immunoblotted. (e) In vitro kinase assays indicating parafibromin is a direct substrate of PTK6. Purified recombinant parafibromin and PTK6 were incubated with 200 μM ATP. The reaction mixtures were subjected to immunoblotting. (f) PTK6 regulates parafibromin/β-catenin interaction. AGS cells were transfected with si-PTK6. TCLs were sequentially immunoprecipitated (IP) with the indicated antibodies. (g) PTK6 knockdown in AGS cells resulted in increased Wnt-reporter activity in the luciferase reporter assay (n=3, mean±s.d. **P<0.01; a two-tailed unpaired Student's t-test). (h–k) Ptk6−/− intestinal organoids show less-differentiated ‘cystic' growth and expanded Wnt signal activation. Immunoblot analysis (h), morphology (i), a percentage of cystic organoids (j) and CD44 immunostaining (k) of control and Ptk6−/−intestinal organoids (n=3, mean±s.d. *P<0.05; Wilcoxon rank sum test). Scale bar, 50 μm.