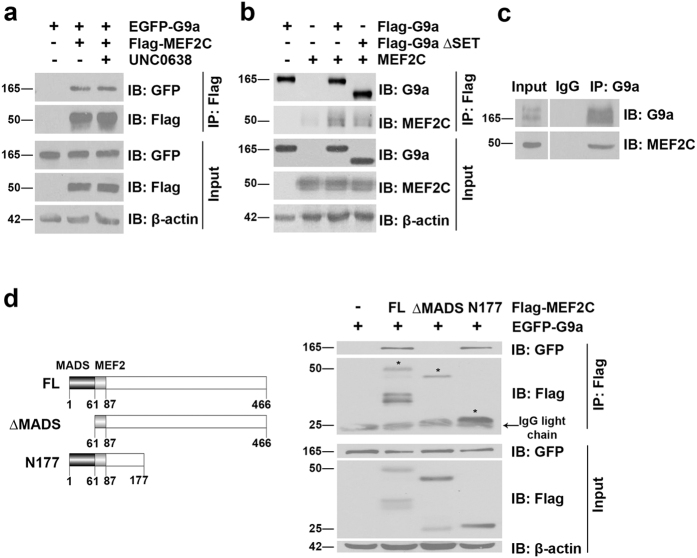
Figure 3. G9a interacts with MEF2C.
(a) Flag-MEF2C and EGFP-G9a were transfected in HEK293T cells which were subsequently treated with UNC0638 for 48 hr. MEF2C was immunoprecipitated with anti-Flag agarose beads and immunoblotted with anti-Flag and anti-GFP antibodies. 10% lysates (Input) were probed for G9a and MEF2C expression. β-actin was used as a loading control. (b) MEF2C was co-transfected with Flag-G9a or Flag-G9a ∆SET in HEK293T cells. G9a was immunoprecipitated using anti-Flag agarose beads and probed for association with MEF2C. 10% lysates were analyzed for G9a and MEF2C expression. (c) Endogenous G9a was immunoprecipitated from C2C12 lysates and probed for association with endogenous MEF2C. IgG was used as a negative control. Input shows expression of G9a and MEF2C in the lysates. (d) Schematic representation of full length MEF2C (FL), MEF2C lacking the MADS domain (∆MADS), and MEF2C with a truncated C-terminus (N177). Numbers indicate amino acid residues on MEF2C. EGFP-G9a was co-expressed with FL, ∆MADS, and N177. MEF2C was immunoprecipitated with anti-Flag agarose beads and lysates were probed for association with G9a using anti-GFP antibody. The expression of full length and MEF2C deletion mutants was analyzed in immunoprecipitates with anti-Flag antibody. Input refers to G9a and MEF2C expression in the lysates. β-actin was used as a loading control. Asterix indicates specific MEF2C bands.