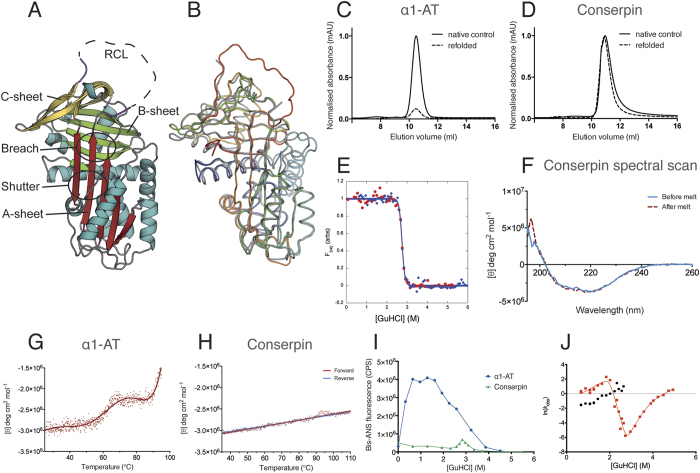
Figure 1. Conserpin conforms to the serpin fold and has superior biophysical properties compared with α1-AT.
(A) Cartoon representation of the 2.4 Å X-ray crystal structure of native conserpin, identifying the breach and shutter regions, the A, B and C sheets (colored in red, green and yellow respectively), and the RCL stumps (magenta). (B) Structural alignment of conserpin (grey) with α1-AT (PDB: 3NE4; spectrum, blue to red). Root mean square deviation (RMSD) = 0.91 Å across 296 backbone Cα atoms. Chemical refolding of (C) α1-AT and (D) conserpin shows that conserpin can refold to a monomer. Chromatograms from a Superdex 75 10/300 size exclusion column are shown. Final protein concentrations loaded onto column were 2 μM. Samples were unfolded in 5 M GuHCl and then diluted out to 0.5 M GuHCl (dotted line). Control samples of native protein are shown as the solid black line. (E) Intrinsic fluorescence equilibrium unfolding (red dots) and refolding (blue diamonds) curves of conserpin coincide, demonstrating reversible folding. (F) CD spectral scans of conserpin before (solid blue line) and after (dashed red line) heating to 110 °C. Variable temperature thermal melts of (G) α1-AT and (H) conserpin as measured by CD at 222 nm. (I) Conserpin shows a significant reduction of intermediate formation during bis-ANS fluorescent equilibrium unfolding of α1-AT (blue circles) and conserpin (green triangles). (J) Kinetic unfolding and refolding experiments. The plot shows the [GuHCl]-dependence of the natural logarithm of the rate constants for unfolding and refolding of conserpin (chevron plot). Two discernable refolding rates are observed (red squares, fast rate; black circles, slower folding rate). The positive slope in each refolding arm suggests the presence of intermediate species that have to partially unfold to reach the native state.