Abstract
Introduction
National adult Tdap vaccination rates are low, reinforcing the need to increase vaccination efforts in primary care offices. The 4 Pillars™ Practice Transformation Program is an evidence-based, step-by-step guide to improving primary care adult vaccination with an online implementation tracking dashboard. This study tested the effectiveness of an intervention to increase adult Tdap vaccination that included the Program, provider education, and one-on-one coaching of practice-based immunization champions.
Methods
25 primary care practices participated in a randomized controlled cluster trial in Year 1 (6/1/2013–5/31-2014) and a pre-post study in Year 2 (6/1/2014–1/31/2015). Baseline year was 6/1/2012–5/31/2013, with data analyzed in 2016. Demographic and vaccination data were derived from de-identified electronic medical record (EMR) extractions. The primary outcomes were vaccination rates and percentage point (PP) changes/year.
Results
The cohort consisted of 70,549 patients ≥ 18 years who were seen in the practices ≥ 1 time each year, with a baseline mean age = 55 years; 35% were men; 56% were non-white; 35% were Hispanic and 20% were on Medicare. Baseline vaccination rate averaged 35%. In the Year 1 RCCT, cumulative Tdap vaccination increased significantly in both intervention and control groups; in both cities, the percentage point increases in the intervention groups (7.7 PP in Pittsburgh and 9.9 PP in Houston) were significantly higher (P<0.001) than in the control groups (6.4 PP in Pittsburgh and 7.6 PP in Houston). In the Year 2 pre-post study, in both cities, active intervention groups increased rates significantly more (6.2 PP for both) than maintenance groups (2.2 PP in Pittsburgh and 4.1 PP in Houston; P<0.001).
Conclusions
An intervention that includes the 4 Pillars™ Practice Transformation Program, staff education and coaching is effective for increasing adult Tdap immunization rates within primary care practices.
Keywords: Tdap vaccine, immunization, adults, pertussis, tetanus
INTRODUCTION
In an effort to stem the rising incidence of pertussis in the United States (U.S.) [1], the Advisory Committee on Immunization Practices (ACIP) first recommended tetanus and diphtheria toxoids and acellular pertussis vaccine (Tdap) vaccination of adults in 2005 for those aged 19–64 years of age who had not received a dose [2]. In 2010, the recommendation was expanded to include adults ≥65 years of age who had not previously received Tdap and who expected to have close contact with an infant less than 12 months old [3], and in 2012 the recommendation was amended to include all adults age ≥19 years [4].
Consequently, national Tdap vaccination rates would be expected to increase in a stepwise fashion with each expansion of the recommendations. In fact, there has been a modest, but steady increase in Tdap vaccination rates. Among younger adults 19–64 years, U.S. national reported rates of Tdap vaccination were 8.2% in 2010 [5], 12.5% in 2011 [5], 15.6% in 2012 [6] and 18.4% in 2013 [7]. In 2012, following the recommendation, uptake was 14.2% [6] for all adults ≥19 years and in 2013 uptake was 17.2% [7]. The recent increases in pertussis cases to more than 28,000 has multiple causes [1], including waning immunity, prevention of infection but not necessarily of transmission by acellular pertussis vaccine, increased reporting, use of better diagnostic tests (i.e., polymerase chain reaction) [8], and modest vaccination coverage [7]. Of these, the modest vaccine coverage is the easiest to address.
The Task Force on Community Preventive Services has recommended multi-strategy, evidence-based interventions [9] as effective means of increasing immunization rates. These interventions should enhance access to vaccination services, increase community demand for vaccines, and improve provider- or system-based interventions.
The 4 Pillars™ Practice Transformation Program (4 Pillars™ Program) is a compilation of evidence-based, best practices and step-by-step guide for increasing adult immunizations in primary care settings with an online implementation tracking dashboard. It is built on decades of research by the investigators into the barriers and facilitators of adult immunizations from the provider and patient perspectives, and trials of successful strategies. The 4 Pillars™ Program was the foundation of a two-year intervention (cluster randomized controlled trial) to increase adult immunization (influenza, pneumococcal and Tdap) rates among patients of primary care practices in two cities. The purpose of this study is to report on changes in adult Tdap immunization rates and factors related to the likelihood of receipt of this vaccine.
METHODS
This trial took place during 2013–2015 and was approved by the Institutional Review Boards of the University of Pittsburgh, Baylor College of Medicine and Harris Health System. The methods have been previously published [10] and are briefly reviewed herein.
Sample Size and Sites
Optimal Design software (University of Michigan, Version 1.77. 2006) was used to calculate sample size, for a cluster randomized trial seeking a 10–15% absolute increase in vaccination rate and a minimum practice size of 100 patients. A sample size of 20 sites was determined to be necessary to achieve 80% power with an alpha of 0.05. Eligible (see below) primary care family and internal medicine practices from a practice-based research network (PBRN) in Pittsburgh (FM Pittnet), a clinical network in Southwestern Pennsylvania (UPMC Community Medicine, Inc.) and a PBRN in Houston (Southern Primary-care Urban Research Network - SPUR-Net) were solicited for participation by identifying practices with adult vaccination rates that were below Healthy People 2020 goals and contacting the practice manager and/or lead physician. When 25 sites agreed to participate, solicitation ceased. All sites used a common electronic medical record (EMR), EpicCare, within their respective health systems.
Cluster Randomization
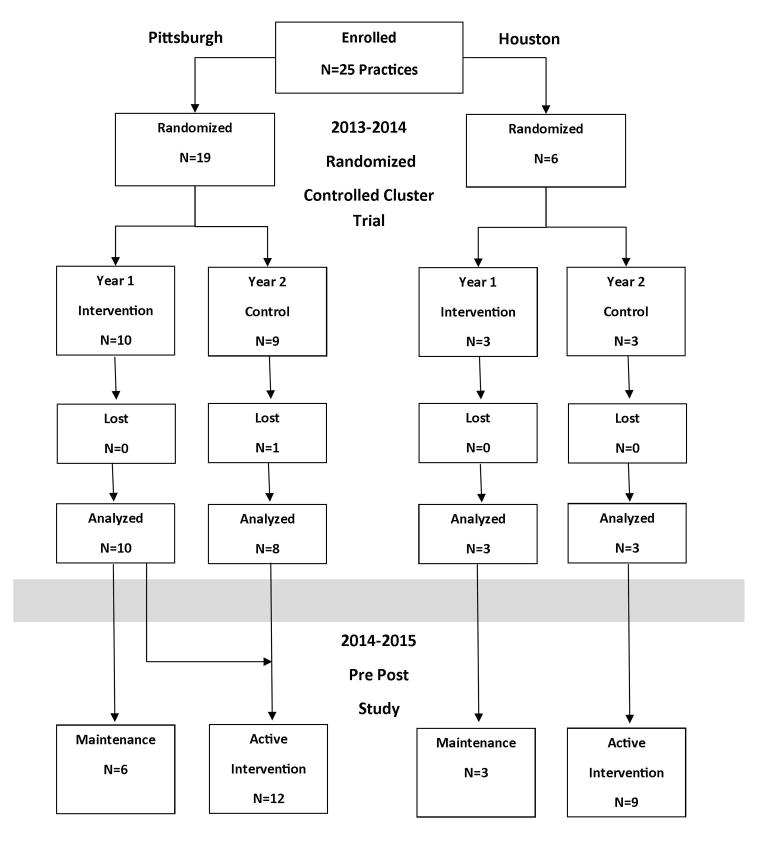
Eligibility requirements for practices included having >100 adult patients, preliminary baseline vaccination rates of <50% for at least one adult vaccine (influenza, pneumococcal, Tdap) and a willingness to make office changes to increase vaccination rates. Participating practices were stratified first by city (Pittsburgh, N=19 and Houston, N=6), then in Pittsburgh, by practice location – urban, suburban and rural, and by discipline (internal vs. family medicine). Houston practices were simply randomized because they were all safety net clinics in the same system. Some practices had more than one site; thus, each site was considered as a cluster for randomization. The practices were then randomized within strata into the Year 1 intervention or Year 2 intervention (controls). Control sites were informed that their intervention would take place the following season and were not contacted again until the next year. The data from Year 1 were analyzed as a RCCT (Figure 1).
Figure 1.
Randomization Scheme - CONSORT
At the end of Year 1, practices were offered the opportunity to continue active intervention during Year 2. Four Pittsburgh practices opted to do so. At the same time, the Year 1 control sites began the intervention. The combined sites that were undergoing the intervention in Year 2 are referred to as the active intervention group. The practices that did not actively participate in Year 2 are referred to the maintenance group. The data from Year 2 were analyzed as a pre-post study.
4 Pillars™ Program
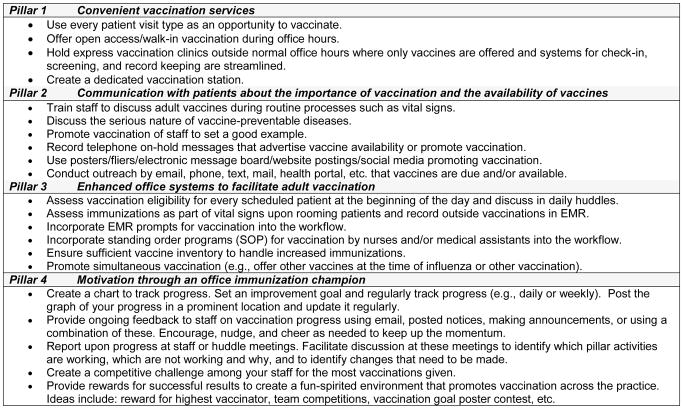
The 4 Pillars™ Practice Transformation Program (4pillarstoolkit.pitt.edu) is founded on four evidence-based [9, 11] key domains: Pillar 1 – Convenient vaccination services; Pillar 2 - Communication with patients about the importance of immunization and the availability of vaccines; Pillar 3 - Enhanced office systems to facilitate immunization; Pillar 4 - Motivation through an office immunization champion (IC). Information in the Figure 2 describes some of the strategies contained in the 4 Pillars™ Program.
Figure 2.
Intervention Strategies Used to Increase Adult Vaccination Rates from the 4 Pillars™ Practice Transformation Program
The 4 Pillars™ Program provided step-by-step guidance for implementing the strategies, and the online practice transformation dashboard showed the practices’ progress through the change process. Each practice was asked to identify an immunization champion (IC) who was responsible for registering the practice and its staff members, and identifying strategies that the practice would implement.
Intervention
The intervention was based on the Diffusion of Innovations theory [12] and has been previously described in detail [10]. Briefly, the intervention included provider education, using the 4 Pillars™ Practice Transformation Program, and one-on-one coaching of the IC for each practice. ICs worked with other staff members or practice leadership to select strategies from each pillar to implement. In addition, each IC was given a graph with goals for vaccine administration based on an overall 20% increase over the previous year’s total adult Tdap vaccinations. These graphs were updated biweekly with actual vaccines given for the IC to monitor progress and encourage the staff to maintain their motivation.
Data collection
De-identified demographic, office visit and vaccination data were derived from EMR data extractions performed by the UPMC Center for Assistance in Research using the eRecord in Pittsburgh and from a similar data extraction by staff of the SPUR-NET for the Houston sites for 6/1/2012 through 5/31/2015. Either the lead physician, nurse or practice manager completed a survey at the end of their active intervention year that assessed the strategies used by sites. There was little variability across sites in the number of strategies used; therefore, this measure was not used in subsequent analyses.
Statistical analyses
The analytic sample consisted of a cohort of patients who had at least one visit in each of the three years with baseline being 6/1/2012–5/31/2013; Year 1 being 6/1/2013–5/31/2014; and Year 2 being 6/1/2014–1/31/2015. Data were analyzed in 2016. The primary outcome measure was the cumulative Tdap vaccination rate reported at the end of baseline, Year 1 and Year 2. Descriptive analyses were performed for patient demographic characteristics (age, sex, race, ethnicity, and health insurance). Data from Pittsburgh and Houston sites were analyzed separately because of differences in patient populations, size and structure of the practices. (See CONSORT scheme in Figure 1.) Age was used as a continuous variable. Health insurance was categorized into Medicaid/self-pay/uninsured, commercial and other insurance, and Medicare. Race and ethnicity were recorded differently in each city. In Pittsburgh sites, with few Hispanic patients, ethnicity was rarely recorded separately from race; hence patients were grouped by race into white and non-white, with blacks and Hispanics assigned to the non-white group; ethnicity data were not analyzed. In Houston sites, with a large proportion of Hispanic patients, race was rarely recorded; hence only ethnicity (Hispanic and non-Hispanic) data are presented and used in analysis. Outcomes were Tdap vaccination rates and percentage point (PP) differences. Proportions were reported for categorical variables and means and standard deviations were reported for continuous variables. Chi-square tests were performed to test for differences in cumulative vaccination rates at different time points.
Year 1 RCCT analyses
To determine which factors were related to Tdap vaccination during the RCCT, while accounting for the clustered nature of the data, Cox proportional hazard models with the robust sandwich estimate were fitted, taking account of heterogeneity in demographic characteristics (including age, sex, race or ethnicity). Models were run ± health insurance, comparing those with Medicare vs. all others because Medicare did not cover the cost of Tdap vaccination.
Year 2 Pre-post analyses
Cox proportional hazard models with the robust sandwich estimate were again fitted, taking account of heterogeneity in demographic characteristics to determine which factors were related to Tdap uptake, comparing vaccination at the end of Year 1 and the end of Year 2. Models were again run ± health insurance.
Statistical significance of two-sided tests was set at a type I error (alpha) equal to 0.05. All analytical procedures were performed using SAS® 9.3.
RESULTS
Demographic characteristics of participating sites and their patients are shown in Table 1. Houston sites were larger practices with higher proportions of Hispanic patients, female patients, and non-commercially insured patients and were all safety net practices, serving primarily economically disadvantaged patients.
Table 1.
Demographic and Practice Characteristics at Baseline
| Site | N | Age, yrs. Mean (SD) | Female, % | White, % | Non-white, % | Hispanic, % | Health insurance status | ||
|---|---|---|---|---|---|---|---|---|---|
| Medicaid,* % | Commercial, % | Medicare, % | |||||||
| Pittsburgh sites | |||||||||
|
| |||||||||
| Intervention | |||||||||
| B | 529 | 65.5 (14.6) | 69.8 | 58.0 | 41.2 | 0.4 | 15.1 | 42.0 | 42.9 |
| C | 2,179 | 60.1 (17.4) | 60.3 | 99.4 | 0.30 | 0.1 | 11.7 | 58.5 | 29.8 |
| D | 3,224 | 66.8 (14.7) | 52.2 | 99.6 | 0.2 | 0.1 | 6.2 | 56.3 | 37.5 |
| E | 1,392 | 56.5 (15.9) | 58.6 | 95.1 | 4.7 | 0.1 | 14.5 | 61.9 | 23.6 |
| G | 417 | 67.0 (14.3) | 52.0 | 82.5 | 16.6 | 0.0 | 5.3 | 49.2 | 45.6 |
| H | 306 | 66.7 (14.9) | 59.2 | 62.4 | 37.0 | 0.0 | 13.7 | 41.5 | 44.8 |
| F | 3,611 | 58.1 (17.0) | 56.8 | 96.4 | 2.4 | 0.3 | 5.0 | 62.6 | 32.4 |
| J | 603 | 62.2(18.6) | 52.7 | 85.9 | 13.3 | 0.2 | 9.0 | 61.4 | 29.7 |
| K | 330 | 56.0(17.7) | 67.6 | 99.1 | 0.3 | 0.3 | 16.4 | 61.5 | 22.1 |
| M | 595 | 66.4(14.9) | 51.1 | 98.0 | 0.2 | 0.3 | 6.7 | 58.8 | 34.5 |
|
| |||||||||
| Total | 13,186 | 61.7 (16.7) | 56.7 | 94.3 | 5.0 | 0.2 | 10.0 | 59.4 | 30.6 |
|
| |||||||||
| Control | |||||||||
| N | 2,102 | 62.0 (16.4) | 58.3 | 6.6 | 0.4 | 0.1 | 8.1 | 67.5 | 24.4 |
| O | 4,324 | 57.2 (16.0) | 53.9 | 98.6 | 0.7 | 0.1 | 7.4 | 65.0 | 27.6 |
| R | 2,534 | 58.8 (14.6) | 52.3 | 97.8 | 1.2 | 0.2 | 4.8 | 67.6 | 27.7 |
| S | 1,045 | 43.6 (16.7) | 75.1 | 53.3 | 45.7 | 0.8 | 58.4 | 23.4 | 18.2 |
| U | 2,612 | 57.1 (17.3) | 63.9 | 90.9 | 7.9 | 0.3 | 11.6 | 53.0 | 35.4 |
| W | 224 | 78.6 (10.4) | 72.8 | 92.4 | 6.3 | 0.9 | 2.2 | 46.0 | 51.8 |
| X | 1,010 | 53.3 (15.0) | 46.6 | 96.5 | 2.0 | 0.0 | 12.0 | 64.5 | 23.6 |
| Y | 3,334 | 60.2 (15.8) | 58.9 | 97.6 | 1.7 | 0.1 | 7.9 | 60.7 | 31.5 |
|
| |||||||||
| Total | 17,185 | 57.8 (16.6) | 57.8 | 94.2 | 5.8 | 0.2 | 11.1 | 60.2 | 28.7 |
|
| |||||||||
| Houston sites | |||||||||
|
| |||||||||
| Intervention | |||||||||
| A | 4,880 | 52.6 (13.7) | 68.8 | 8.0 | 19.7 | 72.3 | 83.8 | 4.8 | 11.5 |
| I | 8,527 | 53.3 (13.7) | 70.7 | 2.7 | 67.6 | 29.6 | 82.9 | 1.5 | 15.6 |
| L | 5,867 | 51.9 (12.0) | 72.6 | 13.1 | 9.3 | 77.6 | 94.5 | .8 | 4.7 |
|
| |||||||||
| Total | 19,274 | 51.0 (13.0) | 72.0 | 6 | 94 | 67 | 86.7 | 2.1 | 11.2 |
|
| |||||||||
| Control | |||||||||
| P | 6,388 | 51.8 (13.4) | 73.0 | 4.1 | 13.9 | 82.0 | 91.7 | 1.1 | 7.1 |
| T | 5,547 | 50.9 (12.9) | 69.5 | 11.1 | 28.7 | 60.2 | 90.8 | 2.3 | 6.9 |
| V | 8,969 | 50.7 (13.2) | 73.7 | 4.0 | 35.6 | 60.3 | 95.1 | 0.6 | 4.3 |
|
| |||||||||
| Total | 20,904 | 53 (13.0) | 71.0 | 7.0 | 93.0 | 55.0 | 92.9 | 1.2 | 5.8 |
|
| |||||||||
| All groups | 70,549 | 55.1 (15.3) | 65.5 | 44.3 | 20.5 | 34.9 | 55.5 | 26.5 | 20.0 |
Note: One site dropped out and was not included in the analyses.
Also includes Other/self-pay/indigent/charity care.
Year 1 RCCT study
Cumulative Tdap vaccination rates at each site and by intervention group at Baseline and Year 1 are shown in Table 2. Individual practice baseline rates for Tdap ranged from a high of 59.5% to a low of 4.2% with average baseline intervention group rates of 34.7% in Pittsburgh 33.1% in Houston for the intervention groups. Control group average rates were slightly higher at 36.4% in Pittsburgh and 35.5% in Houston.
Table 2.
Cumulative Tdap vaccination rates at baseline and the end of the Year 1 randomized controlled cluster trial, by city
| Year 1 – Randomized Controlled Cluster Trial
| |||||||
|---|---|---|---|---|---|---|---|
| Intervention sites | Control sites | ||||||
|
| |||||||
| Site | Total N | % vaccinated by 5/31/2013 | % vaccinated by 5/31/2014 | Site | Total N | % vaccinated By 5/31/2013 | % vaccinated By 5/31/2014 |
| Pittsburgh | |||||||
|
| |||||||
| B | 529 | 16.6 | 21.1 | N | 2,102 | 31.5 | 35.1 |
| C | 2,179 | 42.7 | 52.2 | O | 4,324 | 30.1 | 37.8 |
| D | 3,224 | 12.2 | 15.3 | R | 2,534 | 33.5 | 40.2 |
| E | 1,392 | 19.1 | 22.0 | S | 1,045 | 38.5 | 46.8 |
| F | 3,611 | 59.3 | 70.8 | U | 2,612 | 59.5 | 65.4 |
| G | 417 | 11.2 | 14.1 | W | 224 | 42.8 | 57.1 |
| H | 306 | 4.2 | 5.5 | X | 1,010 | 48.1 | 56.3 |
| J | 603 | 19.5 | 34.3 | Y | 3,334 | 23.4 | 29.7 |
| K | 330 | 6.7 | 9.1 | -- | |||
| M | 595 | 31.7 | 42.2 | -- | |||
|
| |||||||
| Total | 13,186 | 34.7 | 42.4* | Total | 17,185 | 36.4 | 42.8* |
|
| |||||||
| Total percentage point difference from baseline to end of Year 1 | 7.7** | 6.4** | |||||
|
| |||||||
| Houston | |||||||
| A | 4,880 | 45.7 | 56.5 | P | 6,388 | 40.7 | 47.9 |
| I | 8,527 | 20.2 | 24.6 | T | 5,547 | 35.0 | 45.4 |
| L | 5,867 | 41.1 | 58.5 | V | 8,969 | 32.1 | 38.2 |
|
| |||||||
| Intervention total | 19,274 | 33.1 | 43.0* | Control total | 20,904 | 35.5 | 43.1* |
| Total percentage point difference from baseline to end of Year 1 | 9.9† | Total percentage point difference from baseline to end of Year 1 | 7.6† | ||||
|
| |||||||
| All groups | 70,549 | 35.1 | 42.7* | Total percentage point difference from baseline to Year 1 | 7.6 | ||
P<0.001 for difference between Baseline and end of Year 1
P<0.001 for difference between Intervention and Control groups in Pittsburgh
P<0.001 for difference between Intervention and Control groups in Houston
At the end of the Year 1 RCCT, Tdap rates increased significantly in both intervention and control groups in both cities. However, in both cities, the percentage point differences in the intervention groups (7.7 PP in Pittsburgh and 9.9 PP in Houston) were significantly higher (P<0.001) than in the control groups (6.4 PP in Pittsburgh and 7.6 PP in Houston).
In regression analyses (Appendix Table) the likelihood of Tdap vaccination in Year 1 was not related to the intervention. The addition of health insurance to the model did not change the outcomes.
Appendix Table.
Cox proportional hazard models to detect intervention effect on time to vaccination with Tdap adjusting for covariates – Year 1
| Randomized Controlled Cluster Trial Analysis 6/1/2013 – 5/31/2014 | ||||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Pittsburgh | Houston | |||
| Intervention, ref. = Control | 1.12 (0.71–1.76) | 0.630 | 1.36 (0.72–2.56) | 0.342 |
| Female, ref. = Male | 0.88 (0.81–0.95) | 0.002 | 0.93 (0.89–0.98) | 0.003 |
| Age, years | 0.996 (0.989–1.003) | 0.224 | 0.996 (0.987–1.004) | 0.326 |
| White race, ref. = Non-white | 0.99 (0.81–1.23) | 0.987 | -- | -- |
| Non-Hispanic ethnicity, ref. = Hispanic | -- | -- | 0.78 (0.56–1.10) | 0.156 |
Year 2 Pre-Post Study
At the end of the pre-post study comparing the Year 2 active intervention sites and the maintenance sites, individual site Tdap rates ranged from a low of 6.8% to a high of 79.5% (Table 3). In both cities, active intervention groups increased rates significantly more (6.2 PP for both) than maintenance groups (2.2 PP in Pittsburgh and 4.1 PP in Houston; P<0.001). In Cox hazards modeling (Table 4), the likelihood of Tdap vaccination was significantly related to being in the active intervention group (OR=3.72), being male (OR= 0.86 for females), and being younger (OR= 0.99 for age) in Pittsburgh sites and being male (OR=0.99 for females), younger (OR= 0.99 for age) and Hispanic (OR=0.74 for non-Hispanic) in the Houston sites. The addition of health insurance to the models did not affect the likelihood of Tdap vaccination or change the relationships of the other variables to the likelihood of Tdap vaccination in Pittsburgh sites. However, adding health insurance to the model in the Houston sites changed the hazards ratios such that having Medicare insurance lowered the likelihood of Tdap vaccination without changing its relationship to the other variables (data not shown).
Table 3.
Cumulative Tdap vaccination rates at the end of the pre- and post intervention periods for the Year 2 pre-post study.
| Year 2 – Pre-Post Study
| |||||||
|---|---|---|---|---|---|---|---|
| Maintenance Sites | Active Intervention Sites | ||||||
|
| |||||||
| Site | Total N | % vaccinated by 5/31/2014 | % vaccinated by 1/31/2015 | Site | Total N | % vaccinated by 5/31/2014 | % vaccinated by 1/31/2015 |
| Pittsburgh | |||||||
|
| |||||||
| B | 529 | 21.1 | 21.9 | F | 3,611 | 70.8 | 79.5 |
| C | 2,179 | 52.2 | 55.1 | J | 603 | 34.3 | 45.4 |
| D | 3,224 | 15.3 | 17.4 | K | 330 | 9.1 | 11.8 |
| E | 1,392 | 22.0 | 23.8 | M | 595 | 42.2 | 45.3 |
| G | 417 | 14.1 | 17.0 | N | 2,102 | 35.1 | 37.3 |
| H | 306 | 5.5 | 6.8 | O | 4,324 | 37.8 | 43.0 |
| R | 2,534 | 40.1 | 46.2 | ||||
| S | 1,045 | 46.8 | 50.5 | ||||
| U | 2,612 | 65.4 | 70.9 | ||||
| W | 224 | 57.1 | 66.9 | ||||
| X | 1,010 | 56.3 | 67.1 | ||||
| Y | 3,334 | 29.7 | 35.5 | ||||
|
| |||||||
| Total | 13,186 | 24.7 | 26.9* | Total | 17,185 | 43.7 | 49.9* |
| Total percentage point difference from pre- to post study, maintenance sites | 2.2** | Total percentage point difference from pre- to post study, active intervention sites | 6.2** | ||||
|
| |||||||
| Houston | |||||||
| A | 4,880 | 56.5 | 61.8 | P | 6,388 | 47.9 | 53.4 |
| I | 8,527 | 24.6 | 28.7 | T | 5,547 | 45.4 | 53.2 |
| L | 5,867 | 58.4 | 61.5 | V | 8,969 | 38.2 | 43.8 |
|
| |||||||
| total | 19,274 | 43.0 | 47.1* | total | 20,904 | 43.1 | 49.3* |
| Total percentage point difference from pre- to post study, maintenance sites | 4.1† | Total percentage point difference from pre- to post study, active intervention sites | 6.2 | ||||
|
| |||||||
| All groups | 70,549 | 39.6 | 44.7* | Total percentage point difference from pre- to post study, overall | 5.1 | ||
PP= Percentage point difference between pre- and post study.
P<0.001 for PP difference between Maintenance and Active Intervention groups.
Table 4.
Cox proportional hazard models to detect intervention effect on time to vaccination with Tdap adjusting for covariates – Year 2
| Pre-Post Study 6/1/2014 – 1/31/2015
| ||||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P value | Hazard Ratio (95% CI) | P value | |
| Pittsburgh | Houston | |||
| Active intervention, ref. = Maintenance | 3.73 (2.08–6.69) | <0.001 | 1.46 (0.94–2.16) | 0.102 |
| Female, ref. = Male | 0.86 (0.77–0.97) | 0.01 | 0.96 (0.92–0.99) | 0.013 |
| Age, years | 0.99 (0.98–0.996) | 0.003 | 0.99 (0.98–0.99) | <0.001 |
| White race, ref. = Non-white | 1.04 (0.70–1.54) | 0.833 | -- | -- |
| Non-Hispanic ethnicity, ref. = Hispanic | -- | -- | 0.74 (0.58–0.94) | 0.013 |
DISCUSSION
Baseline Tdap vaccination rates in 19 of the 24 practices were higher than the national 2013 reported rate of 17.2% [7], which may be explained by the fact that the rates represented individuals with known access to primary care, even if in a safety net clinic. Previous research of early uptake of Tdap has shown that not having had an office visit in the previous year was related to lower likelihood of Tdap vaccination [13] while low perceived risk of pertussis also explained why many adults had not received the Tdap vaccine [14]. Adults reported increased willingness to receive tetanus and pertussis vaccinations if they were recommended by a physician [15]. Pillar 2 of the 4 Pillars™ Program focuses on increasing patient’s awareness of the availability of vaccines and recommendations to eligible adults by health care providers and staff to receive those vaccines.
Significant increases in Tdap vaccination rates were observed in both intervention and control groups in both cities with significantly larger increases in the intervention groups than in the control groups. Average increases during intervention (either Year 1 or Year 2) ranged from 6.2 to 9.9 percentage points in intervention sites. Recent national data show a 3 percentage point increase among all adults ages 19 years and older [7]. Overall, vaccination rates in primary care practices participating in the intervention increased 12.7 PP over two years, with 21 of 24 practices surpassing the national average rate, suggesting a beneficial effect of the intervention. In a related study, Hawk et al. [16] found that practices that had higher readiness to change characteristics and were considered to be High Implementers had significant increases in Tdap vaccination not observed among practices determined to be Low or Medium Implementers.
In this cohort of adults with a mean age of 55 years, women were less likely to receive the Tdap vaccine than men, and the likelihood of Tdap vaccination decreased with increasing age. These findings may reflect: a) the fact that Tdap recommendations encourage pregnant women to receive Tdap to protect their newborns [3] and these women would typically be a much younger group; and b) the impact of insurance coverage policy that biases against Tdap vaccination among older populations. Under the Affordable Care Act, insurance plans or policies have been required since 2010 to cover the cost of immunization with no cost-sharing by the patient [17]. A notable exception is Medicare, which covers Tdap vaccine through its Part D programs. For older patients who are frequently insured through Medicare, the cost of the vaccine may be a barrier. In the Houston safety net sites, being on Medicare significantly reduced the likelihood of vaccination, supporting the belief that cost is still a barrier to receipt of some vaccines for low income patients. Furthermore, in contrast to typically observed racial disparities in adult vaccination rates [7], likelihood of Tdap vaccination did not differ between whites and non-whites in Pittsburgh and was higher among Hispanic patients than non-Hispanic patients seen in safety net practices in Houston.
Strengths and Limitations
This study’s limitations include significant increases in vaccinations in the control arm when those sites were not in the active intervention; this may be due to a Hawthorne effect, or diffusion of information shared among intervention sites, as has been reported in other studies [18]. Secular trends may partially explain the results but cannot account for the magnitude of these increases. The data came from the EMR only and may not capture outside immunizations; however, the Pennsylvania Statewide Immunization Information System (i.e., registry) is routinely sent to the EMR in a read-only format, from which clinicians can transcribe outside vaccinations. In Houston, the sites were all part of a network wherein, patients could be seen and vaccinated in any of the offices. They could have been exposed to intervention efforts at an intervention site, but received the vaccine later at a control site, as there is considerable movement among practices in this network. The vaccination would have been attributed to whichever site was considered the patient’s medical home. Thus, the “credit” for vaccination may have been erroneously applied.
Based on feedback given to the research assistant who interacted with each practice’s immunization champion, implementation issues were noted in practices whose increases were less than 10 PP. For example, one Year 1 intervention site did not fully implement the study and another site served a community in which many patients deny vaccination based on religious tenets (i.e., Amish). The lowest performing sites were in the Year 1 RCCT intervention group, whose feedback on vaccination rates was delayed. During the Year 1 intervention, ICs infrequently used the dashboard reporting that the website was not user-friendly. Revisions to the website and timely feedback from the EMR resolved these issues in Year 2.
On the other hand, this is the first study to focus on increasing Tdap vaccinations among adults in primary care since its universal recommendation for all adults. Its strengths are its randomized design, the large number and diversity of patients, diverse practice settings, including safety net clinics, cumulative vaccination reporting, real-world implementation, and analysis of maintenance of rates post intervention. These factors support the generalizability of the intervention. In another study, we have reported that practices with specific characteristics are able to engage more fully in the intervention resulting in larger increases in vaccination rates [16].
Conclusions
Clinically and statistically significant improvements in Tdap vaccination rates were achieved in diverse primary care practices, including safety net clinics serving disadvantaged Hispanics, using an intervention that includes the 4 Pillars™ Practice Transformation Program and its online practice transformation dashboard. These changes were maintained in the post-intervention period.
Highlights.
There is little research on interventions to improve Tdap vaccination among adults.
The 4 Pillars Immunization Toolkit is a step-by-step guide for primary care practices.
Tdap uptake increased significantly in intervention and control groups.
Tdap increases were higher in intervention than maintenance groups in Year 2.
Primary care practices can successfully increase Tdap uptake using the 4 Pillars Toolkit.
Acknowledgments
Funding Sources
This work was supported by the Centers for Disease Control and Prevention [grant number U01 IP000662] and the National Institutes of Health [grant numbers UL1 RR024153 and UL1TR000005].
Footnotes
Clinical Trial Registry Name/Number: NCT01868334
Conflict of Interest:
Drs. Zimmerman and Lin have a research grant from Sanofi Pasteur, Inc. Drs. Zimmerman, Nowalk and Lin have research grants from Merck & Co, Inc., and Pfizer, Inc. Dr. Middleton is a lecturer for Sanofi Pasteur and Merck & Co. and a physician advisor for Sanofi Pasteur, Pfizer, Inc. and Merck & Co. The other authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Pertussis Outbreak Trends. [ http://www.cdc.gov/pertussis/outbreaks/trends.html]
- 2.Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, et al. Preventing Tetanus, Diphtheria, and Pertussis Among Adults: Use of Tetanus Toxoid, Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine. Morbidity and Mortality Weekly Report (MMWR) 2006;55:1–33. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Vaccine From the Advisory Committee on Immunization Practices, 2010. Morbidity and Mortality Weekly Report (MMWR) 2011;60(1):13–15. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Vaccine in Adults Aged 65 Years and Older-Advisory Committee on Immunization Practices. morbidity and Mortality Weekly Report (MMWR) 2012;61(25):468–470. [PubMed] [Google Scholar]
- 5.Williams WW, Lu PJ, Greby S, Bridges CB, Ahmed F, Liang JL, Pilishvili T, Hales C. Noninfluenza Vaccination Coverage Among Adults - United States, 2011. Morbidity and Mortality Weekly Report (MMWR) 2013;62(4):66–72. [PMC free article] [PubMed] [Google Scholar]
- 6.Williams WW, Lu PJ, O’Halloran A, Bridges CB, Pilishvili T, Hales CM, Markowitz LE. Noninfluenza Vaccination Coverage Among Adults - United States, 2012. Mmwr-Morbid Mortal W. 2014;63(5):95–102. [PMC free article] [PubMed] [Google Scholar]
- 7.Williams WW, Lu PJ, O’Halloran A, Bridges CB, Kim DK, Pilishvili T, Hales CM, Markowitz LE. Vaccination Coverage Among Adults, Excluding Influenza Vaccination - United States, 2013. Mmwr-Morbid Mortal W. 2015;64(4):95–102. [PMC free article] [PubMed] [Google Scholar]
- 8.Tan T, Dalby T, Forsyth K, Halperin SA, Heininger U, Hozbor D, Plotkin S, Ulloa-Gutierrez R, von König CHW. Pertussis across the globe: recent epidemiologic trends from 2000 to 2013. The Pediatric infectious disease journal. 2015;34(9):e222–e232. doi: 10.1097/INF.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 9.Guide to Community Preventive Services. [ http://www.thecommunityguide.org/index.html]
- 10.Zimmerman RK, Brown AE, Pavlik VN, Moehling KK, Raviotta JM, Lin CJ, Zhang S, Hawk M, Kyle S, Patel S, et al. Using the 4 Pillars™ Immunization Toolkit to increase pneumococcal immunizations for older adults: A cluster randomized trial. Journal of the American Geriatrics Society. 2016 doi: 10.1111/jgs.14451. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melinkovich P, Hammer A, Staudenmaier A, Berg M. Improving pediatric immunization rates in a safety-net delivery system. Joint Commission journal on quality and patient safety / Joint Commission Resources. 2007;33(4):205–210. doi: 10.1016/s1553-7250(07)33024-9. [DOI] [PubMed] [Google Scholar]
- 12.Oldenburg B, Parcel SG. Diffusion of Innovations. In: Glanz Karen, Rimer BK, Lewis FM., editors. Health Behavior and Health Education. 3. San Francisco: John Wiley and Sons, Inc; 2002. pp. 312–334. [Google Scholar]
- 13.Johns TL, Roetzheim R, Chen R. Predictors of Tetanus–Diphtheria– Acellular Pertussis Vaccination Among Adults Receiving Tetanus Vaccine in the United States: Data From the 2008 National Health Interview Survey. Journal of Primary Care & Community Health. 2013;4(2):95–100. doi: 10.1177/2150131912455428. [DOI] [PubMed] [Google Scholar]
- 14.Miller BL, Kretsinger K, Euler GL, Lu P-J, Ahmed F. Barriers to early uptake of tetanus, diphtheria and acellular pertussis vaccine (Tdap) among adults—United States, 2005–2007. Vaccine. 2011;29(22):3850–3856. doi: 10.1016/j.vaccine.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 15.Skowronski DM, Pielak K, Remple VP, Halperin BA, Patrick DM, Naus M, McIntyre C. Adult tetanus, diphtheria and pertussis immunization: knowledge, beliefs, behavior and anticipated uptake. Vaccine. 2004;23(3):353–361. doi: 10.1016/j.vaccine.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 16.MH, Nowalk MP, KKM, JMR, VNP, AEB, RKZ, Ricci EM. Using a mixed methods approach to examine practice characteristics associated with implementation of an adult immunization intervention using the 4 Pillars™ Immunization Toolkit. J Healthcare Qual. 2016 doi: 10.1097/JHQ.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh HK, Sebelius KG. Promoting Prevention through the Affordable Care Act. New Engl J Med. 2010;363(14):1296–1299. doi: 10.1056/NEJMp1008560. [DOI] [PubMed] [Google Scholar]
- 18.Pavlik VN, Chan W, Hyman DJ, Feldman P, Ogedegbe G, Schwartz JE, McDonald M, Einhorn P, Tobin JN. Designing and Evaluating Health Systems Level Hypertension Control Interventions for African-Americans: Lessons from a Pooled Analysis of Three Cluster Randomized Trials. Current hypertension reviews. 2015;11(2):123–131. doi: 10.2174/1573402111666150325234503. [DOI] [PMC free article] [PubMed] [Google Scholar]