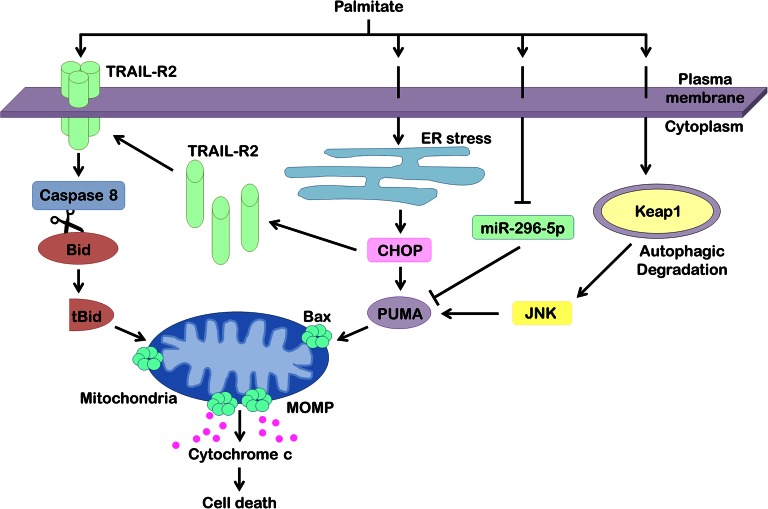
Fig. 3.
Apoptotic signaling networks in hepatocyte lipotoxicity. Hepatocyte treatment with palmitate results in ligand-independent clustering and activation of the TRAIL-R2, causing caspase-dependent cell death. Active caspase 8 cleaves Bid into its truncated form (tBid), which translocates to mitochondria to promote release of proapoptotic factors, such as cytochrome c. Palmitate also induces ER stress, which upregulates proapoptotic PUMA and TRAIL-R2 via transcription factor CHOP. Increased levels of PUMA facilitate hepatocyte apoptosis via the mitochondrial pathway. Palmitate has also been found to cause cellular depletion of miR-296-5p, a microRNA that, via complementary binding to the 3′-UTR of PUMA mRNA, causes its degradation. Loss of miR-296-5p during lipotoxicity removes this break on PUMA posttranscriptional regulation, thereby increasing cellular levels of PUMA. Finally, palmitate induces autophagic degradation of Keap1, resulting in JNK activation and PUMA upregulation. Along with other proapoptotic inputs, PUMA sensitizes the hepatocyte to Bax activation and MOMP culminating in cell death.