Abstract
Background
5-Fluorouracil (5-FU) based treatment is the standard therapy for metastatic colorectal cancer (CRC), but the development of chemoresistance is inevitable. Increasing evidence shows that dysregulation of microRNAs (miRNAs) is involved in malignant transformation. Thus, it is imperative that we find new diagnostic and prognostic marker for chemotherapy in CRC.
Material/Methods
For clinical parameter analysis, 78 CRC tissues and adjacent normal tissues and 45 serum specimens from CRC patients were included in this study. For chemo-response analysis, 116 primary tissues were collected from the patients receiving first-line 5-FU treatment. Quantitative Real-Time PCR (qRT-PCR) was used to detect microRNAs expression.
Results
The expression of miR-429 was significantly increased in both serum and primary tissues from CRC patients, and enhanced miR-429 level was associated with tumor size, lymph node metastasis, and TNM stage. The diagnostic and prognostic values were also confirmed in CRC by using primary tissues. For patients receiving 5-FU-based treatment, miR-429 levels were significantly lower in responding group. The proportions of patients that did not experience response to therapy were higher in primary tumors with high miR-429 expression levels as compared with primary tumors with low miR-429 expression levels. Finally, Kaplan-Meier survival analysis showed that miR-429 is an independent prognostic indicator for chemo-response to 5-FU therapy among CRC patients.
Conclusions
High level of miR-429 expression was correlated with enhanced malignant potential and poor prognosis of CRC patients. Furthermore, miR-429 could affect the chemo-sensitivity of CRC patients to 5-FU therapy and was associated with poor response to 5-FU-based chemotherapy in patients with CRC.
MeSH Keywords: Antineoplastic Agents, Colorectal Neoplasms, Fluorouracil, MicroRNAs
Background
Colorectal cancer (CRC) is a leading cause of cancer-related deaths in the world. It is the second and third-most commonly diagnosed cancer in females and males respectively, and more than 1.2 million patients are diagnosed with CRC every year [1,2]. Currently, CRC patients with lymph node metastasis (TNM stage III) are treated with adjuvant chemotherapy that includes cytotoxic drugs such as 5-fluorouracil (5-FU) and oxaliplatin, following surgical resection of the cancer [3,4]. However, it is far from being the perfect treatment. Large proportion of patients receiving chemotherapy finally became metastatic and chemo-resistant, and this has been a key barrier to the efficacy of CRC treatment [5–7]. Thus, finding new diagnostic and prognostic targets will be indispensable for developing effective therapy for CRC patients.
Recently, a number of adjuvant strategies have also been developed to further enhance the response and survival rates. Despite the advances in diagnostic methods, such as fecal occult blood testing and stool DNA tests, early diagnosis for CRC still remains difficult and the overall survival rate of CRC patients has not changed dramatically [8]. At present, more and more researchers focus on non-invasive biomarkers, and one or a cluster of specific marker is urgently needed for increasing the early detection rate pf CRC and decreasing the mortality rate of CRC [9]. Predictive biomarkers are better if they are blood-based, as blood is easily available and provides the chance to monitor chemotherapy response. Therefore, it is important to identify blood markers that predict a patient’s responsiveness to chemotherapy, which may allow for the development of targeted therapies for overcoming chemoresistance.
MicroRNAs (miRNAs) are increasingly recognized to be key regulators of gene expression in several biological systems, including cancer [10,11]. They regulate gene expression primarily via their interaction with the 3′UTRs of target mRNAs, resulting in mRNA decay or translational repression [12]. MiRNAs in CRC tissue and serum have been reported to be of prognostic significance [13]. Liu et al. demonstrated that miR-1260b is a potential prognostic biomarker in CRC [14]. MiRNAs sometime exert a role as oncogenes or tumor suppressor genes through affecting the response to various therapeutic regimens. In recent years, studies have highlighted the association between miRNAs and response to some tumors [15]. These findings suggest that miRNA can act as important regulators during chemoresistance among different cancers.
Previous reports showed that miR-429 was important for predicting clinical outcome in gastric cancer patients through suppressing tumor cell proliferation and inhibiting tumor metastasis [16,17]. However, another study revealed that miR-429 was an oncogene and predicted poor survival in ovarian cancer patients [18]. These findings suggest that miR-429 may be a oncogene or tumor suppressor in different conditions. Recently, Cantini et al. found that miR-429 has prognostic value in CRC [12], but its role in CRC chemotherapy was still unknown. In this study, we intended to determine the miR-429 expression levels in primary tissues and serum from CRC patients, and further analyzed its association with pathologic factors. Moreover, the potential diagnostic and prognostic value in patients receiving 5-FU-based chemotherapy was also investigated. The results indicated that miR-429 expression was significantly downregulated in CRC patients and can serve as a diagnostic and prognostic factor in CRC patients receiving first-line 5-FU-based treatment.
Material and Methods
Patients and samples
For clinical parameter analysis, 78 CRC tissues and paired normal tissues were collected at the Second People’s Hospital of Hefei City. Fresh surgical specimens were immediately frozen in liquid nitrogen and were then stored at −80°C until further use. At the same time, pre-operative blood samples were collected from another 45 patients with CRC, as well as from 45 healthy volunteers for further analysis. Immediately after collection, the blood samples were processed for isolation of cell-free nucleic acids to prevent contamination from cellular nucleic acids. Serum samples were then stored at −80°C until further processing. For the chemo-response study, 116 primary tissues were collected from the patients who received standard 5-FU-based chemotherapy at the Second People’s Hospital of Hefei City between 2009 and 2013. All the patients were pathologically confirmed as CRC patients and the clinical samples were collected before chemotherapy was started. Patients were classified according to the WHO criteria and staged according to the tumor-node-metastasis (TNM) classification. Tumor response status was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 criteria and was assigned to patients with complete or partial response (CR and PR, respectively) and stable or progressive disease (SD and PD, respectively) in tumor measurements confirmed by repeat studies performed no less than four weeks after the criteria for response was first met. Overall survival was updated on February 1, 2012 and was defined as the time from inclusion in the study until death for any reason. All patients received standardized follow-up including CT-scans of the chest and abdomen at 12 months and 36 months after surgery. Written informed consent was obtained from all patients according to local ethical regulations of the Ethics Committee of the Second People’s Hospital of Hefei City.
Total RNA extraction
Total RNA of CRC tissues and adjacent tissues was extracted by using TRIzol (Invitrogen, USA); and plasma RNA was extracted by using acid phenol according to the manufacturer’s instructions. Total RNA was quantified by microfluidics analysis (Gene Quant, Switzerland). The samples with A260 nm/A280 nm ratios between 1.8 and 2.0 were used for further experiments.
Quantitative Real-Time PCR (qRT-PCR)
For primary CRC tissues, the cDNA was synthesized from 200 ng extracted total RNA using the PrimeScript RT reagent Kit (Takara Bio Company, Shiga, Japan) and amplified by qRT-PCR with SYBR Green Kit (Takara Bio Company) on 7500 RealTime PCR system (Applied Biosystems). For serum miR-429 detection, we treated the total RNA with a reverse transcription kit (Bioteck, Beijing, China) according to the manufacturer’s protocol to obtain cDNA and then qRT-PCR was performed by using 7500 RealTime PCR system (Applied Biosystems). The 2−ΔCt method was used to determine the relative quantification of gene expression levels and U6 was used as a housekeeping gene. All reactions were performed in triplicate.
Statistical analysis
For CRC serum versus healthy control, and CRC tissue versus adjacent non-tumor tissue, differences in mean expression were determined using Mann-Whitney U test. The association between tissue and plasma miRNA levels was analyzed using the Spearman’s correlation coefficient. The survival curves of CRC patients were estimated via the Kaplan-Meier method and the difference in survival curves was estimated using log-rank testing. A p value <0.05 was considered statistically significant. Statistical analyses were undertaken using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA, USA).
Results
Expression of miR-429 in CRC tissues relative to adjacent non-tumorous tissues
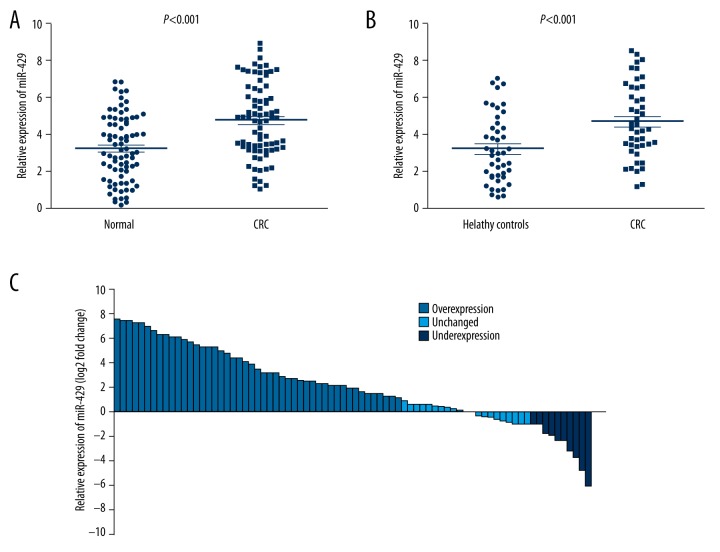
Seventy-eight patients who provide primary tissue samples were enrolled in this study. Among these patients, there were 49 males and 29 females; 35 patients were older than 60 years of age, and 43 patients were younger than 60 years of age with the median age of 56 years old, range (31–79 years). According to the seventh edition of the tumor node metastasis (TNM) staging criteria of CRC, 8 cases were considered stage I, 34 cases were stage II, 29 cases were stage III, and 7 cases were stage IV. qRT-PCR was used to examine the miR-429 levels in 78 cancerous and paired noncancerous tissues, and our results indicated that the expression of miR-429 was significantly increased in tumor tissues compared with paired normal tissues (p<0.001, Figure 1A). Moreover, the miR-429 expression in 60.3% (47 of 78) cases were 2-fold higher than in adjacent tissues (Figure 1B).
Figure 1.
MiR-429 expression was significantly increased in CRC serum and primary tissues. (A) miR-429 expression was significantly increased in CRC primary tissues compared with adjacent normal tissues. (B) 60.3% (47 of 78) cases had at least 2-fold higher expression of miR-429 in the CRC tissues compared with adjacent normal tissues. (C) The expression of miR-429 was significantly increased in serum of CRC patients compared with healthy individuals.
We then investigated to verify the correlation between miR-429 and clinicopathological characters. As shown in Table 1, miR-429 expression was positively correlated with TNM stage, lymph node metastasis, distant metastasis and tumor size, while no correlations were observed between miR-429 expression and age, sex, tumor location, and differentiation. These results suggest that high expression of miR-429 may participate in the carcinogenesis of CRC.
Table 1.
Clinical characteristics of 78 patients and the expression of miR-429 in CRC tissues.
| Factors | Case | MiR-429 Median (range) |
P |
|---|---|---|---|
| Gender | 0.826 | ||
| Male | 49 | 4.81 (1.12–8.65) | |
| Female | 29 | 4.98 (1.24–7.87) | |
| Age(years) | 0.537 | ||
| <60 | 43 | 4.64 (1.24–6.77) | |
| ≥60 | 35 | 4.99 (1.26–7.71) | |
| Tumor size | 0.036 | ||
| <6 cm | 49 | 3.89 (1.24–7.23) | |
| ≥6 cm | 29 | 5.02 (2.26–8.98) | |
| Tumor location | 0.198 | ||
| Colon | 37 | 4.64 (1.45–7.46) | |
| Rectum | 41 | 5.05 (2.12–7.77) | |
| Differentiation | 0.218 | ||
| Well | 27 | 4.77 (1.45–6.65) | |
| Moderate | 33 | 4.56 (2.30–7.10) | |
| Poor | 18 | 5.32 (3.11–8.17) | |
| Local invasion | 0.063 | ||
| T1–T2 | 27 | 4.38 (1.12–7.65) | |
| T3–T4 | 51 | 4.99 (2.98–8.76) | |
| Lymph node metastasis | 0.001 | ||
| No | 42 | 3.36 (1.26–7.38) | |
| Yes | 36 | 5.71 (3.31–8.78) | |
| Distant metastasis | 0.000 | ||
| No | 71 | 3.56 (1.38–7.02) | |
| Yes | 7 | 5.56 (2.99–8.98) | |
| TNM stage | |||
| I–II | 42 | 3.32 (1.26–6.15) | 0.000 |
| III–IV | 36 | 5.98 (2.78–8.43) | |
MiRNAs expression in serum of CRC patients and healthy volunteers
To further verify the clinical value of miR-429 in diagnosis of CRC, we tested the expression level of miR-429 in serum of CRC patients. Another independent set of 45 serum samples from cancer patients (32 males and 13 females) were enrolled with the median age of 58 years old (range 40–72 years). The corresponding data were following: stage I (5 cases), II (19 cases), III (18 cases) and IV (3 cases); well differentiation (18 cases), moderately differentiation (17 cases) and poor differentiation (10 cases). Forty-five serum samples from healthy individuals (27 males and 18 females) were used as controls, and the median age of controls was 47 years old (range 25–69). As shown in Figure 1C, the serum miR-429 expression levels in CRC patients were also statistically significantly higher than healthy controls (p<0.01). We also analyzed the association of serum miR-429 with clincopathological factors. Different from the primary tissues, serum miR-429 level was significantly correlated with TNM stage, but not correlated with other factors such as age, sex, tumor location, tumor size, local invasion, lymph node metastasis, differentiation, and distant metastasis (Table 2).
Table 2.
Clinical characteristics of 45 patients and the expression of miR-429 in CRC serum.
| Factors | Case | MiR-429 Median (range) |
P |
|---|---|---|---|
| Gender | 0.904 | ||
| Male | 32 | 4.21 (1.15–7.91) | |
| Female | 13 | 4.53 (1.26–8.01) | |
| Age(years) | 0.772 | ||
| <60 | 30 | 4.24 (2.11–7.12) | |
| ≥60 | 15 | 4.57 (1.01–7.87) | |
| Tumor size | 0.351 | ||
| <6 cm | 12 | 4.13 (1.24–7.23) | |
| ≥6 cm | 33 | 4.66 (2.12–8.32) | |
| Tumor location | 0.212 | ||
| Colon | 21 | 4.14 (1.35–8.46) | |
| Rectum | 24 | 4.78 (2.00–7.98) | |
| Differentiation | 0.373 | ||
| Well | 18 | 4.43 (2.11–7.55) | |
| Moderate | 17 | 4.37 (2.45–7.91) | |
| Poor | 10 | 4.98 (3.08–8.01) | |
| Local invasion | 0.084 | ||
| T1–T2 | 19 | 4.03 (2.01–7.12) | |
| T3–T4 | 26 | 4.76 (2.45–8.33) | |
| Lymph node metastasis | 0.378 | ||
| No | 24 | 4.26 (2.08–7.56) | |
| Yes | 21 | 4.63 (2.48–7.55) | |
| Distant metastasis | 0.060 | ||
| No | 42 | 4.29 (1.34–6.99) | |
| Yes | 3 | 5.04 (2.11–8.54) | |
| TNM stage | |||
| I–II | 24 | 3.98 (1.10–6.72) | 0.034 |
| III–IV | 21 | 5.32 (2.48–8.39) | |
MiR-429 is a diagnostic indicator for CRC patients
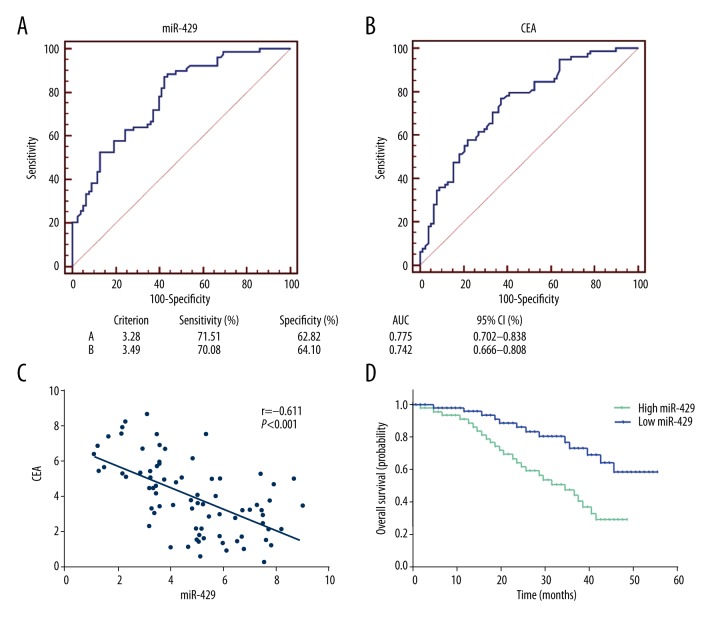
We next sought to validate the diagnostic role of miR-429 of primary CRC tissues by using the receiver operating characteristic (ROC) curve analysis, and compared it with the traditional CRC marker, CEA. As shown in Figure 2A and 2B, the area under the curve (AUC) of miR-429 were 0.775 (95% CI: 0.702–0.838). The diagnostic sensitivity and specificity was 71.790% and 62.820%, respectively. The AUC of miR-429 was higher than CEA, while its AUC was 0.742 (95% CI: 0.666–0.808), and diagnostic sensitivity and specificity were 70.51% and 64.10%, respectively. And there is no statistical significance of AUC between miR-429 and CEA. Besides, the expression of miR-429 was negatively correlated with CEA (Figure 2C). These data showed that miR-429 had a potential diagnostic value for CRC patients.
Figure 2.
The diagnostic and prognostic value of miR-429 in CRC tissues. ROC curve was drawn to exhibit the diagnostic capacity of miR-429 (A) and CEA (B). (C) miR-429 expression was negatively correlated with CEA in primary CRC tissues. (D) Patients with high expression of miR-429 were associated with shorter overall survival compared with patients with low expression.
MiR-429 predicts poor survival in CRC patients
We also evaluated the role of miR-429 in prognosis of CRC patients. We divided these patients into a high and a low expression group by using the median value (4.89) of 78 primary CRC tissues. The 5-year survival rate of the CRC patients whose tumors expressed high levels of miR-429 was 35.9% (14/39), which was significantly lower than that of the patients whose tumors expressed low levels of miR-429 (66.7%, 26/39). More importantly, the Kaplan-Meier analysis indicated that patients with high expression of miR-429 was associated with shorter overall survival compared with the low expressing CRC patients (p=0.0021, Figure 2D).
Low miR-429 expression was associated with positive response to 5-FU-based treatment in CRC patients
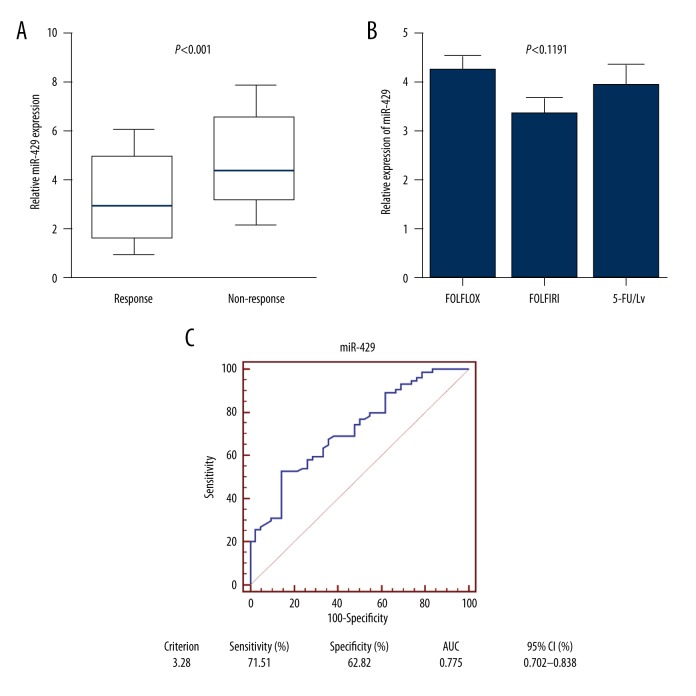
We next sought to validate the association between miR-429 and response to treatment in 116 primary tissues. Patients were divided into responding (CR+PR) and non-responding (SD+PD) groups according to RECIST criteria. The expression level of miR-429 was significantly higher in patients who did not respond to treatment (n=42) compared with patients responding to treatment (n=74) (p<0.001, Figure 3A). To exclude the influence from different chemotherapy methods, miR-429 expression was determined in patients receiving different 5-FU-based regimens, and the results showed that no difference was found between different regimens, including FOLFOX (5-FU+oxaliplatin+leucovorin); FOLFIRI (5-FU+leucovorin+irinotecan) and 5-FU/Lv (5-FU+leucovorin) (p=0.1191, Figure 3B). Then, a receiver operating characteristic (ROC) curve analysis was performed to investigate the potential significance in predicting the patients’ response to chemotherapy. The area under the curve of miR-429 were 0.721 (95% CI: 0.630–0.800, Figure 3C), and the diagnostic sensitivity and specificity reached 52.70% and 85.71%, respectively. These results indicated that miR-429 was associated with 5-FU-based chemo-response among CRC patients.
Figure 3.
MiR-429 expression was associated with chemo-response to 5-FU-based treatment in CRC patients. (A) MiR-429 levels were significantly higher in non-responding group than in responding group among CRC patients receiving 5-FU-based chemotherapy. (B) No significant difference was found of miR-429 level among different 5-FU-based chemotherapy methods. (C) ROC curve was drawn to explore the capacity of miR-429 in distinguishing responding and non-responding patients receiving 5-FU-based treatment.
MiR-429 is a prognostic factor in patients receiving 5-FU-based treatment
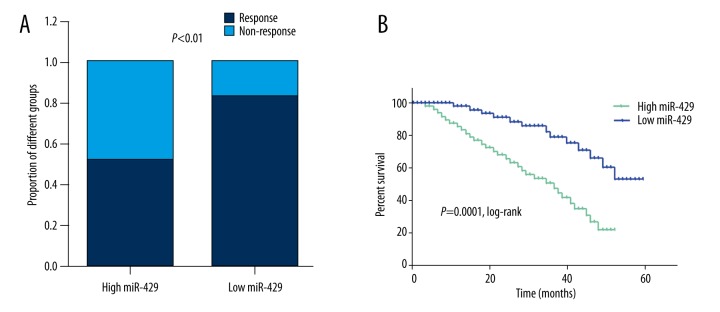
Finally, we aimed to explore the prognostic value of miR-429 for chemotherapy. When we stratified patients into a low (n=46) and a high (n=70) groups with the previously established optimal cut-off value of relative miR-429 level (3.08), the proportion of patients not responding to chemotherapy was significantly higher in the high miR-429 expressing group than in the low group (p<0.01, Figure 4A). The 5-year survival rate of the CRC patients whose tumors expressed high levels of miR-429 was 21.4% (15/70), which was significantly lower than that of the patients whose tumors expressed low levels of miR-429 (52.2%, 24/46); this difference was statistically significant (p=0.0011). Besides, the Kaplan-Meier survival analysis also showed that patients with a low level of miR-429 expression had a significant longer overall survival than did those with a high level of miR-429 expression (p=0.0001, Figure 4B). Furthermore, we performed Cox regression multivariate analysis to identify whether miR-429 was an independent indicator for overall survival of CRC patients who received 5-FU chemotherapy. As expected, a high miR-429 level was significantly correlated with poorer survival in an independent manner (Table 3). Taken together, these results indicated that miR-429 was an independent predictor for chemo-response to 5-FU-based treatment in CRC patients.
Figure 4.
MiR-429 is a prognostic factor in patients receiving 5-FU-based treatment. (A) The proportion of CRC patients not responding to 5-FU-based chemotherapy was significantly higher in the high miR-429 expressing group than in the low group. (B) Patients with high miR-429 expression were associated with short overall survival among CRC patients receiving 5-FU-based therapy.
Table 3.
Multivariate Cox proportional hazards regression model analysis for overall survival in CRC patients receiving 5-FU based treatment.
| Factors | Multivariate analysis | ||
|---|---|---|---|
| RR | 95% CI | P | |
| Gender | 0.999 | 0.498–2.003 | 0.998 |
| Age | 1.932 | 0.879–4.246 | 0.091 |
| Tumor location | 0.792 | 0.402–1.827 | 0.435 |
| Tumor size | 0.918 | 0.423–1.998 | 0.831 |
| Differentiation | 1.013 | 0.514–1.887 | 0.921 |
| Local invasion | 1.275 | 0.614–2.731 | 0.571 |
| Lymph node metastasis | 1.804 | 1.023–2.937 | 0.036 |
| Distant metastasis | 2.047 | 1.018–3.918 | 0.021 |
| TNM stage | 3.772 | 1.309–9.385 | 0.011 |
| MiR-429 expression | 2.296 | 1.105–4.528 | 0.027 |
Discussion
Despite recent chemotherapeutic regimens that have significantly increased survival in metastatic disease, invariably, nearly all CRC patients finally become chemo-resistant [5]. It is urgent to find new diagnostic methods and prognostic targets for CRC. In this study, we validated the upregulation of miR-429 among CRC specimens and serum samples, and found that enhanced miR-429 expression was correlated with tumor progression in CRC. Moreover, we found that high miR-429 expression was negatively associated with chemotherapy response in CRC patients receiving 5-FU-based chemotherapy.
MicroRNAs, acting as post-transcriptional regulators of gene expression, can regulate 30% of the protein coding genes in the human genome [12,19]. Several studies have identified miR-429 as an oncogene that is involved in the development in early stages of malignancies. It is reported to promote tumor progression in pancreatic cancer [20]; and downregulation of miR-429 was found to significantly inhibited cell growth of endometrial tumors [21]. Recently, Cristobal et al. reported that deregulation of miR-429 indicated its potential relevant role in patients with colorectal cancer liver metastasis, which further indicating the functional role of miR-429 in CRC [22]. Consistent with previous studies, our data showed that the miR-429 levels increased statistically significantly in CRC specimens compared with corresponding adjacent non-tumorous tissue. We also found that enhanced miR-429 expression was associated with tumor progression, such as TNM stage. Besides, the ROC curves revealed a comparative diagnostic value of miR-429 when compared with CEA, a traditional biomarker in CRC. These findings indicated that miR-429 could be a diagnostic indicator for CRC patients.
It is widely accepted that the effective ways to improve the recovery rate and the prognosis of cancer patients are early detection and early treatment [23]. Therefore, it is very important to search for cell-free markers for early diagnosis and prognosis evaluation. MicroRNA stability is a prerequisite for potential tumor markers [24]. Utilizing miRNA expression level in peripheral blood to diagnose tumors early is effective and deserves to be explored further because miRNA is very stable in blood plasma and serum. Thus, we wondered whether miR-429 is also highly expressed in peripheral blood of CRC patients. Our results indicated that miR-429 levels were also significantly increased in serum from CRC patients compared with healthy controls, which further validated the potential function of miR-429 in CRC patients.
5-fluorouracil is an antimetabolite used as the first-line chemotherapeutic agent for various cancers. However, patients who respond to chemotherapy initially will eventually acquire resistance to these treatments, and the mechanisms underlying such acquired chemoresistance remain unclear. Elucidating the mechanisms of chemoresistance is critical for development of effective therapeutic strategies. The identification of cancer-specific miRNAs is critical for understanding the roles of miRNAs in tumorigenesis and may be important for defining novel therapeutic targets [25,26]. Karaayvaz et al. revealed that enhanced miR-129 expression in CRC promoted cell death and improved chemosensitivity to 5-FU treatment [27]. Moreover, Liu et al. found that miR-429 was an oncogene regulated by evodiamine and berberine in human CRC. These studies indicated that miR-429 might play important roles during chemotherapy in CRC [28]. In our research, our novel results indicated that miR-429 was significantly correlated with 5-FU response in CRC patients, and exerted its important function in distinguishing the patients with a response to 5-FU treatment from the patients with no response. Moreover, high miR-429 level was positively associated with objective response in CRC patients receiving 5-FU-based chemotherapy. These results revealed a diagnostic value of miR-429 for 5-FU-based chemotherapy in CRC patients.
We also investigated the prognostic function of miR-429 in CRC. CRC patients who expressed high miR-429 levels showed a shorter 5-year survival rate than patients with low miR-429 levels. These data, together with further multivariate analyses, suggest that high expression of miR-429 might be a significant independent predictor of poor prognosis for CRC patients. Additionally, the survival analysis indicated that overall survival of CRC patients with high level miR-429 expression who received 5-FU-based chemotherapy was significantly lower than that of patients with low-level miR-429 expression. Thus, it was concluded that overexpression of miR-429 might be involved in 5-FU resistant phenotypes of human CRCs.
A limitation of the current study is a lack of a large cohort of samples to establish a strong correlation between the expression of miR-429 and chemo-response to 5-FU-based treatments among CRC patients. Future studies with a large cohort of samples based on a multi-center, randomized controlled trial are needed to help identify potential clinical applications of miR-429 in CRC patients. Besides, this article focused only on individual phenomena, and further explanation of the underlying mechanism (e.g., miRNA target genes) or mechanistic pathways are needed in future studies.
Conclusions
In conclusion, this study showed that high miR-429 expression levels in CRC tissues and serum was associated with enhanced malignant potential and poor prognosis of CRC patients. Furthermore, miR-429 could affect the chemo-sensitivity of CRC patients to 5-FU and was associated with poor response to 5-FU-based chemotherapy in CRC patients. Thus, miR-429 may be a novel prognostic biomarker and therapeutic target for CRC patients. Suppression of miR-429 could be a future direction to enhance chemo-sensitivity to 5-FU-based chemotherapy regimen.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Source of support: This study was supported by the National Natural Science Foundation of China (No.G09H020002)
References
- 1.Han D, Wang M, Ma N, et al. Long noncoding RNAs: Novel players in colorectal cancer. Cancer Lett. 2015;361:13–21. doi: 10.1016/j.canlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Li PL, Zhang X, Wang LL, et al. MicroRNA-218 is a prognostic indicator in colorectal cancer and enhances 5-fluorouracil-induced apoptosis by targeting BIRC5. Carcinogenesis. 2015;36:1484–93. doi: 10.1093/carcin/bgv145. [DOI] [PubMed] [Google Scholar]
- 3.Shah MA, Renfro LA, Allegra CJ, et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: Analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) Database. J Clin Oncol. 2016;34(8):843–53. doi: 10.1200/JCO.2015.63.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andre T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: Updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–87. doi: 10.1200/JCO.2015.63.4238. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Landriscina M, Maddalena F, Laudiero G, Esposito F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid Redox Signal. 2009;11:2701–16. doi: 10.1089/ars.2009.2692. [DOI] [PubMed] [Google Scholar]
- 7.Sau A1, Pellizzari Tregno F, Valentino F, et al. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 2010;500:116–22. doi: 10.1016/j.abb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Lu YF, Liu ZC, Li ZH, et al. Esophageal/gastric cancer screening in high-risk populations in Henan Province China. Asian Pac J Cancer Prev. 2014;15(3):1419–22. doi: 10.7314/apjcp.2014.15.3.1419. [DOI] [PubMed] [Google Scholar]
- 9.Tsujiura M, Ichikawa D, Komatsu S, et al. Circulating microRNAs in plasma of patients with gastric cancers. Br J Cancer. 2010;102(7):1174–79. doi: 10.1038/sj.bjc.6605608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409(6818):363–66. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantini L, Isella C, Petti C, et al. MicroRNA-mRNA interactions underlying colorectal cancer molecular subtypes. Nat Commun. 2015;6:8878. doi: 10.1038/ncomms9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101(9):2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu DR, Guan QL, Gao MT, et al. miR-1260b is a potential prognostic biomarker in colorectal cancer. Med Sci Monit. 2016;22:2417–23. doi: 10.12659/MSM.898733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schetter AJ, Okayama H, Harris CC. The role of microRNAs in colorectal cancer. Cancer J. 2012;18(3):244–52. doi: 10.1097/PPO.0b013e318258b78f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun T, Wang C, Xing J, Wu D. miR-429 modulates the expression of c-myc in human gastric carcinoma cells. Eur J Cancer. 2011;47(17):2552–59. doi: 10.1016/j.ejca.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Uhlmann S, Zhang JD, Schwager A, et al. miR-200bc/429 cluster targets PLCgamma1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene. 2010;29(30):4297–306. doi: 10.1038/onc.2010.201. [DOI] [PubMed] [Google Scholar]
- 18.Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14(9):2690–95. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 19.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353(17):1768–71. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 20.Mees ST, Mardin WA, Wendel C, et al. EP300 – a miRNA-regulated metastasis suppressor gene in ductal adenocarcinomas of the pancreas. Int J Cancer. 2010;126(1):114–24. doi: 10.1002/ijc.24695. [DOI] [PubMed] [Google Scholar]
- 21.Leskela S, Leandro-Garcia LJ, Mendiola M, et al. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr-Relat Cancer. 2011;18(1):85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 22.Cristóbal I, Rincón R, Manso R, et al. Deregulation of miR-200b, miR-200c and miR-429 indicates its potential relevant role in patients with colorectal cancer liver metastasis. J Surg Oncol. 2014;110(4):484–85. doi: 10.1002/jso.23661. [DOI] [PubMed] [Google Scholar]
- 23.Xia L, Ren Y, Fang X, et al. Prognostic role of common microRNA polymorphisms in cancers: Evidence from a meta-analysis. PLoS One. 2014;9(10):e106799. doi: 10.1371/journal.pone.0106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood based markers for cancer detection. Proc Natl Acad Sci USA. 2008;105(30):10513–18. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu F, Deng H, Yao H, et al. Mir-30 reduction maintains self-renewal and inhibits apoptosis in breast tumor-initiating cells. Oncogene. 2010;29(29):4194–204. doi: 10.1038/onc.2010.167. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Huang H, Sun L, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15(12):3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 27.Karaayvaz M, Zhai H, Ju J. miR-129 promotes apoptosis and enhances chemosensitivity to 5-fluorouracil in colorectal cancer. Cell Death Dis. 2013;4:e659. doi: 10.1038/cddis.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu H, Huang C, Wu L, Wen B. Effect of evodiamine and berberine on miR-429 as an oncogene in human colorectal cancer. Onco Targets Ther. 2016;9:4121–27. doi: 10.2147/OTT.S104729. [DOI] [PMC free article] [PubMed] [Google Scholar]