Figure 2.
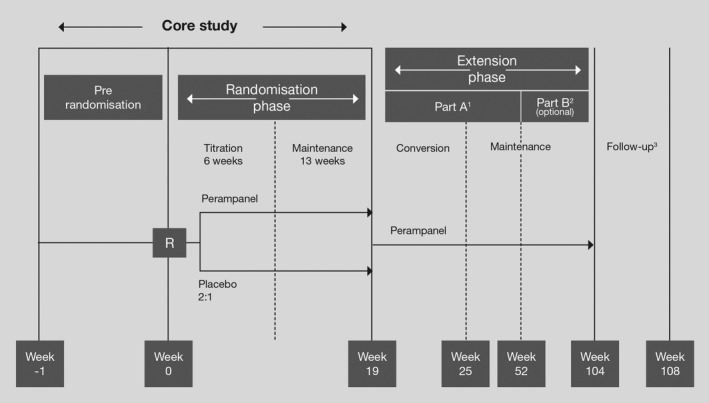
Design of a phase 2 study of perampanel (study 235) (Hussein et al. 2015; Pina‐Garza et al. 2015; Renfroe et al. 2015). R, randomization. 1All patients were retained to the last visit of extension part A. 2Part B was optional (a patient proceeded to or completed part B if perampanel was not commercially available or extended‐access program 401 was not in place in their country of residence). 3Follow‐up was conducted for all patients 4 weeks after their last on‐treatment visit.
