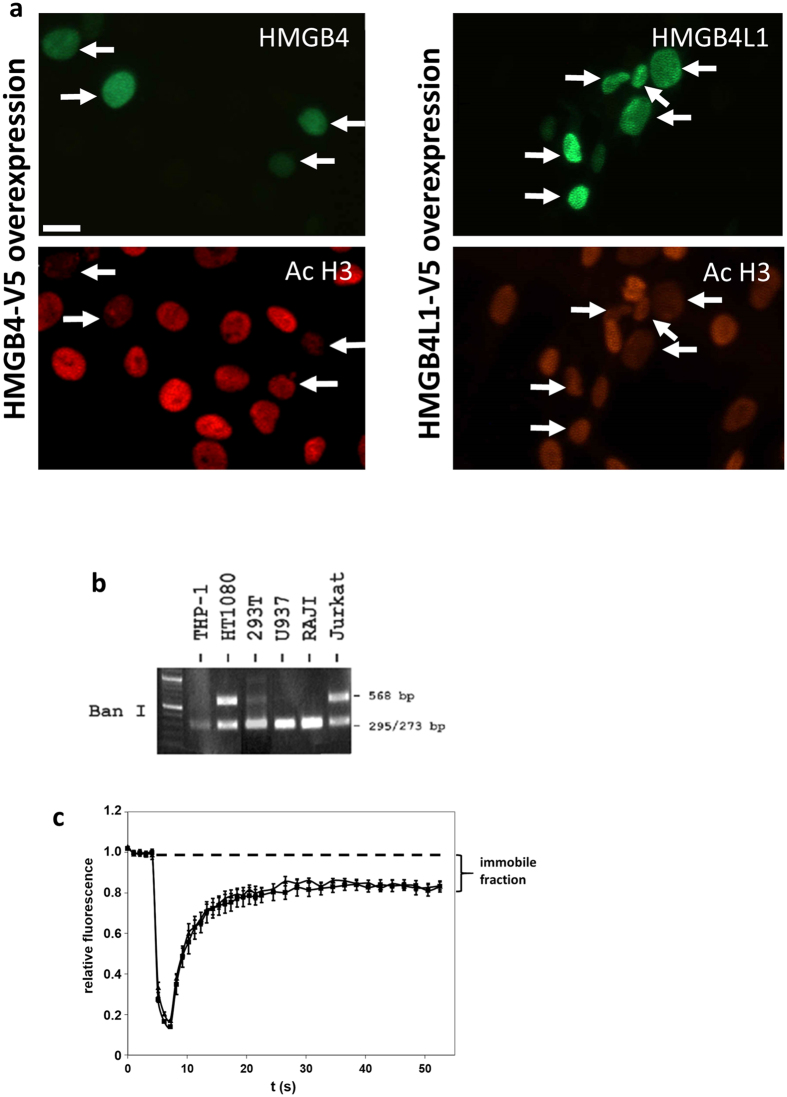
Figure 2. Nuclear localization of HMGB4 and HMGB4L1.
(a) Rat glioblastoma C6 –cells transiently transfected with V5-tagged HMGB4 and HMGB4L1 were double immunostained with anti-V5-tag and anti-acetylated K9/K14 histone H3 antibodies. Immunofluorescence staining intensity of anti-acetylated K9/K14 histone H3 is reduced in C6 –cells transiently expressing HMGB4 or HMGB4L1. Arrows indicate HMGB4- and HMGB4L1-positive nuclei in double immunostained cells. Scale bar 20 = μm. (b) Single nucleotide polymorphism of the human HMGB4 gene. Genomic DNA of different human cell lines was amplified in PCR with primers specific to HMGB4 gene and subjected to Ban 1 restriction enzyme analysis. SNP rs10379 destroys Ban 1 cleavage site in the coding sequence of HMGB4 gene. Gel figure indicates that HT1080 and Jurkat cells are heterozygotes for rs10379 SNP. (c) Nuclear mobility FRAP analyses of wild type HMGB4 and rs10379 polymorphic human HMGB4 protein forms. NIH-3T3 -cells were transfected with an expression vector coding for the prominent allele or the polymorphic allele of human HMGB4-EGFP fusion proteins. One brightly fluorescent area in the nucleus with high expression of HMGB4-EGFP was bleached with three laser pulses and recovery of fluorescence was measured. Values from pre-bleach areas were determined as 1 and normalized values for bleached areas were calculated. The failing of full fluorescent recovery indicates the existence of an immobile fraction of HMGB4-EGFP in the nucleus. Triangles = wild type HMGB4, squares = SNP form of HMGB4; n = 5; ± SD.